Abstract
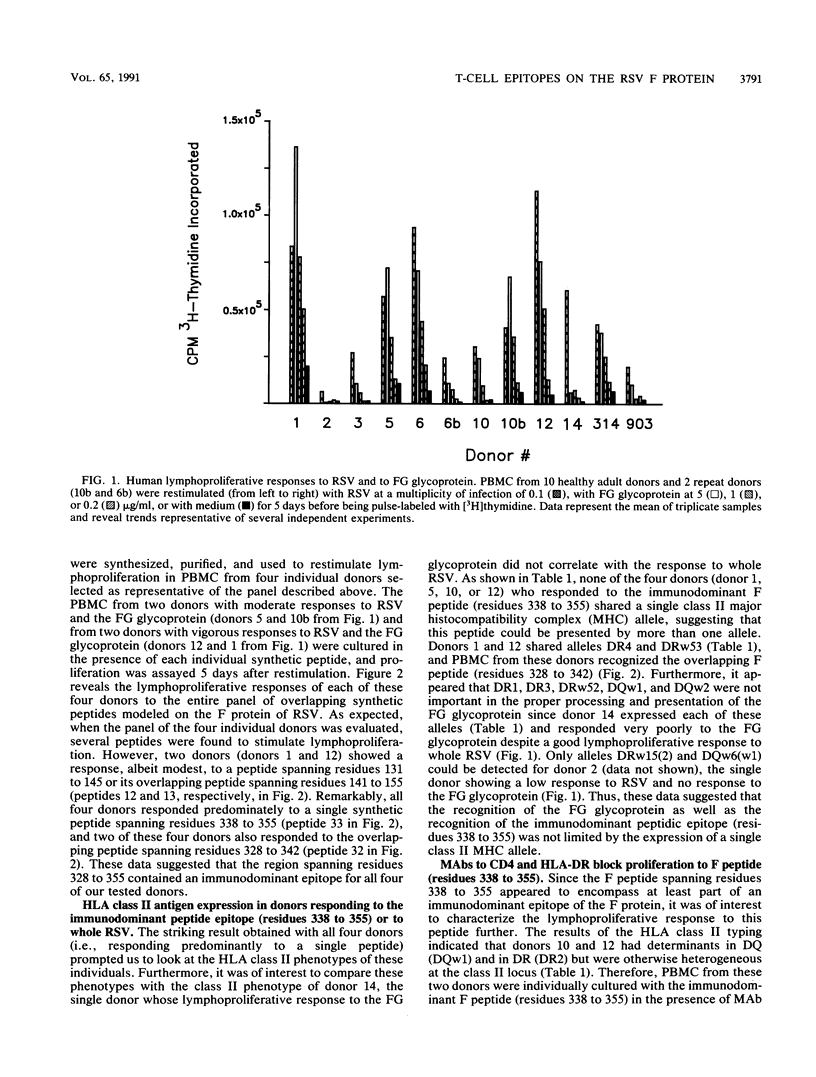
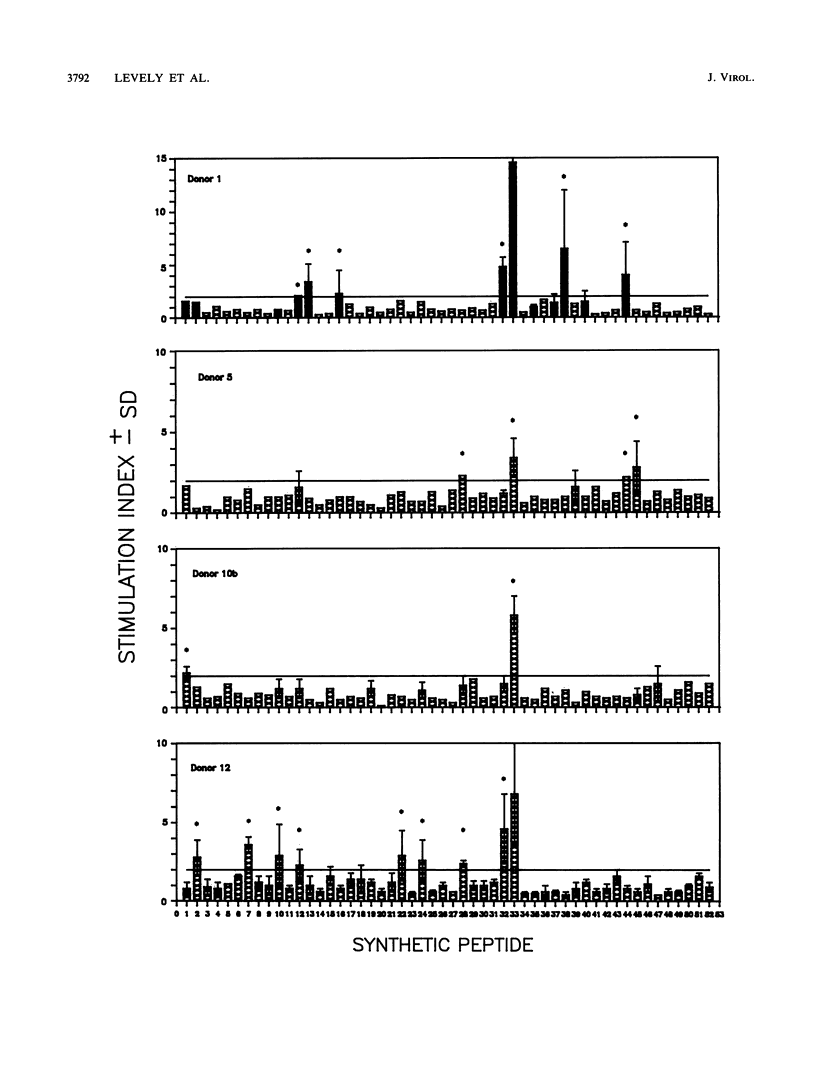
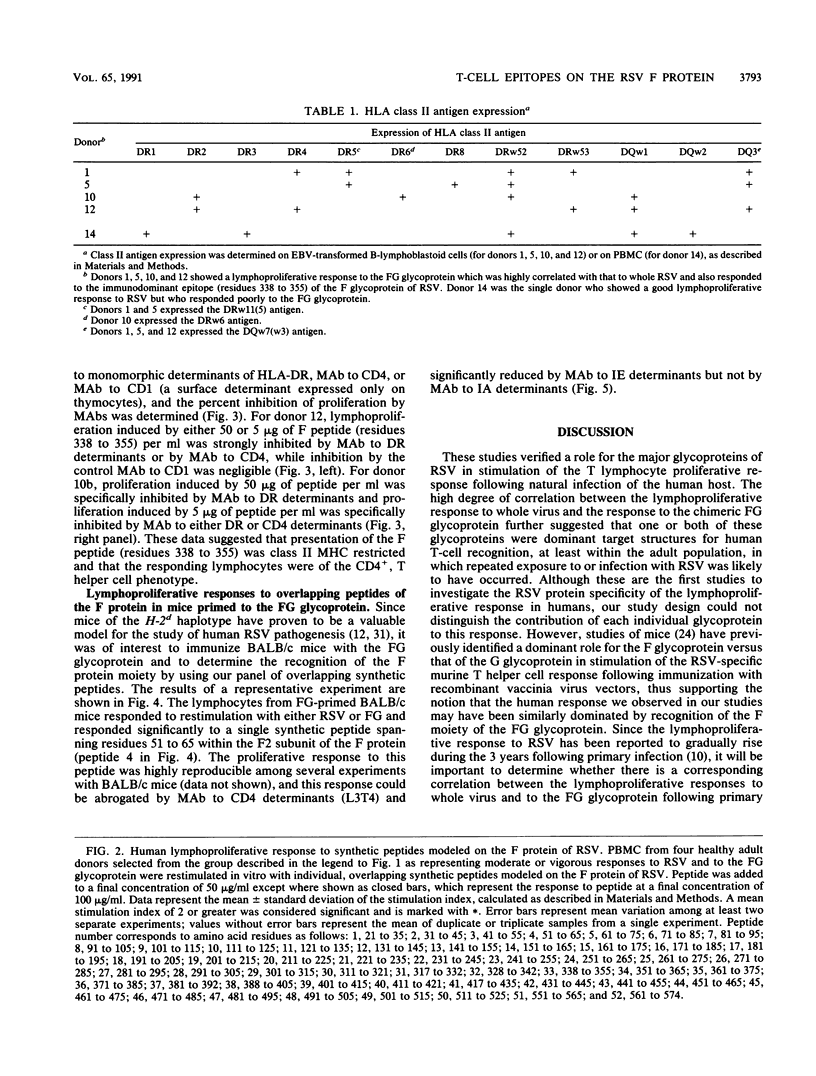
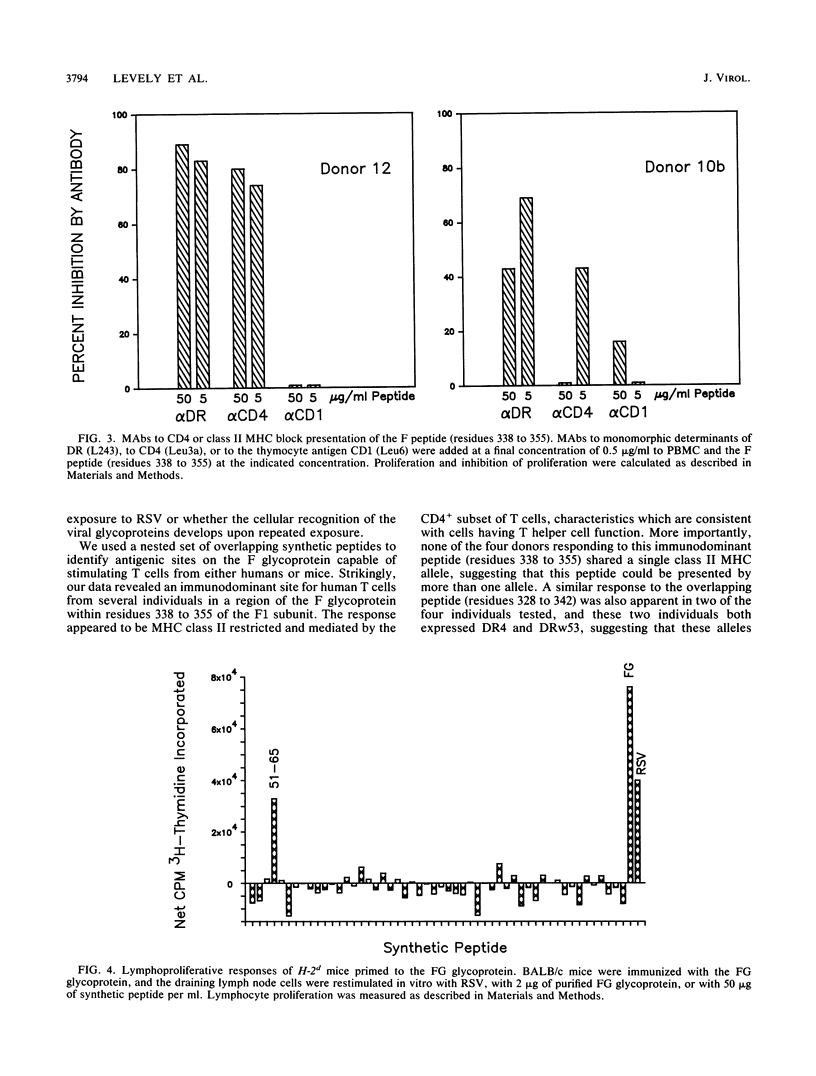
The lymphocyte proliferative responses to respiratory syncytial virus (RSV) were evaluated for 10 healthy adult donors and compared with proliferative responses to a chimeric glycoprotein (FG glycoprotein) which consists of the extracellular domains of both the F and G proteins of RSV and which is produced from a recombinant baculovirus. The lymphocytes of all 10 donors responded to RSV, and the proliferative responses to the whole virus were highly correlated with the responses to the FG glycoprotein. These data suggested that one or both of these glycoproteins of RSV were major target structures for stimulation of the human lymphocyte proliferative response among virus-specific memory T cells. The lymphocytes of four donors were evaluated further for their proliferative responses to a nested set of overlapping peptides modeled on the extracellular and cytoplasmic domains of the F protein of RSV. Strikingly, the lymphocytes of all 4 donors responded primarily to a region defined by a single peptide spanning residues 338 to 355, and the lymphocytes of 2 donors responded to an overlapping peptide spanning residues 328 to 342 also, thus defining a region of the F1 subunit within residues 328 to 355 that may circumscribe an immunodominant site for stimulation of human T cells from a variety of individuals. This region of the F protein is highly conserved among A and B subgroup viruses. As revealed by monoclonal antibody blocking studies, the lymphocytes responding to this antigenic site had characteristics consistent with T helper cells. Similar epitope mapping studies were performed with BALB/c mice immunized with the FG protein in which a relatively hydrophobic peptide spanning residues 51 to 65 within the F2 subunit appeared to be the major T cell recognition determinant. The data are discussed with respect to an antigenic map of the F protein and the potential construction of a synthetic vaccine for RSV.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlind-Stopner B., Utter G., Mufson M. A., Orvell C., Lerner R. A., Norrby E. A subgroup-specific antigenic site in the G protein of respiratory syncytial virus forms a disulfide-bonded loop. J Virol. 1990 Oct;64(10):5143–5148. doi: 10.1128/jvi.64.10.5143-5148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. J., Bingham P., Hierholzer J. C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol. 1988 Nov;62(11):4232–4238. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Stone Y. O., Tsou C., Fernie B. F. Identification of epitopes on respiratory syncytial virus proteins by competitive binding immunoassay. J Clin Microbiol. 1986 Mar;23(3):475–480. doi: 10.1128/jcm.23.3.475-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Beeler J. A., van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989 Jul;63(7):2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Walters R. R., Stier M. A., Wathen M. W. Protection of cotton rats against human respiratory syncytial virus by vaccination with a novel chimeric FG glycoprotein. J Gen Virol. 1989 Oct;70(Pt 10):2637–2644. doi: 10.1099/0022-1317-70-10-2637. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Prince G. A., Murphy B. R., Venkatesan S., Chanock R. M., Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald G. W., Almond J. R., Henderson F. W. Cellular and humoral immunity in recurrent respiratory syncytial virus infections. Pediatr Res. 1983 Sep;17(9):753–758. doi: 10.1203/00006450-198309000-00014. [DOI] [PubMed] [Google Scholar]
- García-Barreno B., Palomo C., Peñas C., Delgado T., Perez-Breña P., Melero J. A. Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J Virol. 1989 Feb;63(2):925–932. doi: 10.1128/jvi.63.2.925-932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. S., Perkins M. D., Wright P. F., Karzon D. T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988 Oct;26(2):153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. Respiratory syncytial virus mRNA coding assignments. J Virol. 1983 May;46(2):667–672. doi: 10.1128/jvi.46.2.667-672.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T., Chu E., Geha R. S. Antigen presentation by human B cells: T cell proliferation induced by Epstein Barr virus B lymphoblastoid cells. J Immunol. 1982 Oct;129(4):1446–1450. [PubMed] [Google Scholar]
- Johnson P. R., Jr, Olmsted R. A., Prince G. A., Murphy B. R., Alling D. W., Walsh E. E., Collins P. L. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987 Oct;61(10):3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. W., Leikin S. L., Arrobio J., Brandt C. D., Chanock R. M., Parrott R. H. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976 Jan;10(1):75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- Levine S., Klaiber-Franco R., Paradiso P. R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987 Sep;68(Pt 9):2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- López J. A., Peñas C., García-Barreno B., Melero J. A., Portela A. Location of a highly conserved neutralizing epitope in the F glycoprotein of human respiratory syncytial virus. J Virol. 1990 Feb;64(2):927–930. doi: 10.1128/jvi.64.2.927-930.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. A., Rubino K. L., Levely M. E., Adams E. G., Collins P. L. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990 Sep;64(9):4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Mufson M. A., Alexander H., Houghten R. A., Lerner R. A. Site-directed serology with synthetic peptides representing the large glycoprotein G of respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6572–6576. doi: 10.1073/pnas.84.18.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Elango N., Prince G. A., Murphy B. R., Johnson P. R., Moss B., Chanock R. M., Collins P. L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P. J., Anderson K., Wertz G. W., Askonas B. A. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990 Apr;64(4):1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P. J., Pemberton R. M., Ball L. A., Wertz G. W., Askonas B. A. Helper T cell recognition of respiratory syncytial virus in mice. J Gen Virol. 1988 Feb;69(Pt 2):305–312. doi: 10.1099/0022-1317-69-2-305. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E., Mufson M. A. Preparation and characterization of monoclonal antibodies directed against five structural components of human respiratory syncytial virus subgroup B. J Gen Virol. 1987 Dec;68(Pt 12):3125–3135. doi: 10.1099/0022-1317-68-12-3125. [DOI] [PubMed] [Google Scholar]
- Pemberton R. M., Cannon M. J., Openshaw P. J., Ball L. A., Wertz G. W., Askonas B. A. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987 Aug;68(Pt 8):2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- Scopes G. E., Watt P. J., Lambden P. R. Identification of a linear epitope on the fusion glycoprotein of respiratory syncytial virus. J Gen Virol. 1990 Jan;71(Pt 1):53–59. doi: 10.1099/0022-1317-71-1-53. [DOI] [PubMed] [Google Scholar]
- Scott R., Kaul A., Scott M., Chiba Y., Ogra P. L. Development of in vitro correlates of cell-mediated immunity to respiratory syncytial virus infection in humans. J Infect Dis. 1978 Jun;137(6):810–817. doi: 10.1093/infdis/137.6.810. [DOI] [PubMed] [Google Scholar]
- Scott R., Pullan C. R., Scott M., McQuillin J. Cell-mediated immunity in respiratory syncytial virus disease. J Med Virol. 1984;13(1):105–114. doi: 10.1002/jmv.1890130112. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hughes M., Collins A. P. Respiratory syncytial virus infection in mice. Infect Immun. 1984 Feb;43(2):649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M., Nadon F., Seguin C., Dionne G., Lacroix M. Identification of a synthetic peptide as part of a major neutralization epitope of respiratory syncytial virus. J Gen Virol. 1987 Sep;68(Pt 9):2273–2280. doi: 10.1099/0022-1317-68-9-2273. [DOI] [PubMed] [Google Scholar]
- Trudel M., Nadon F., Séguin C., Ghoubril S., Payment P., Trépanier P. Immunovirological studies on human respiratory syncytial virus structural proteins. Can J Microbiol. 1986 Jan;32(1):15–21. doi: 10.1139/m86-004. [DOI] [PubMed] [Google Scholar]
- Trudel M., Nadon F., Séguin C., Payment P., Talbot P. J. Respiratory syncytial virus fusion glycoprotein: further characterization of a major epitope involved in virus neutralization. Can J Microbiol. 1987 Oct;33(10):933–938. doi: 10.1139/m87-164. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Cote P. J., Fernie B. F., Schlesinger J. J., Brandriss M. W. Analysis of the respiratory syncytial virus fusion protein using monoclonal and polyclonal antibodies. J Gen Virol. 1986 Mar;67(Pt 3):505–513. doi: 10.1099/0022-1317-67-3-505. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Hall C. B., Briselli M., Brandriss M. W., Schlesinger J. J. Immunization with glycoprotein subunits of respiratory syncytial virus to protect cotton rats against viral infection. J Infect Dis. 1987 Jun;155(6):1198–1204. doi: 10.1093/infdis/155.6.1198. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Hall C. B., Schlesinger J. J., Brandriss M. W., Hildreth S., Paradiso P. Comparison of antigenic sites of subtype-specific respiratory syncytial virus attachment proteins. J Gen Virol. 1989 Nov;70(Pt 11):2953–2961. doi: 10.1099/0022-1317-70-11-2953. [DOI] [PubMed] [Google Scholar]
- Walsh E. E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983 Jul;47(1):171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984 Feb;43(2):756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Schlesinger J. J., Brandriss M. W. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus. J Gen Virol. 1984 Apr;65(Pt 4):761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Brideau R. J., Thomsen D. R. Immunization of cotton rats with the human respiratory syncytial virus F glycoprotein produced using a baculovirus vector. J Infect Dis. 1989 Feb;159(2):255–264. doi: 10.1093/infdis/159.2.255. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Brideau R. J., Thomsen D. R., Murphy B. R. Characterization of a novel human respiratory syncytial virus chimeric FG glycoprotein expressed using a baculovirus vector. J Gen Virol. 1989 Oct;70(Pt 10):2625–2635. doi: 10.1099/0022-1317-70-10-2625. [DOI] [PubMed] [Google Scholar]


