Abstract
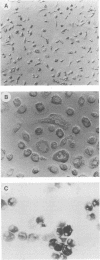
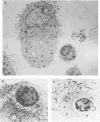
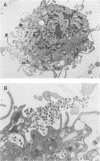
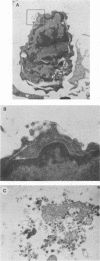
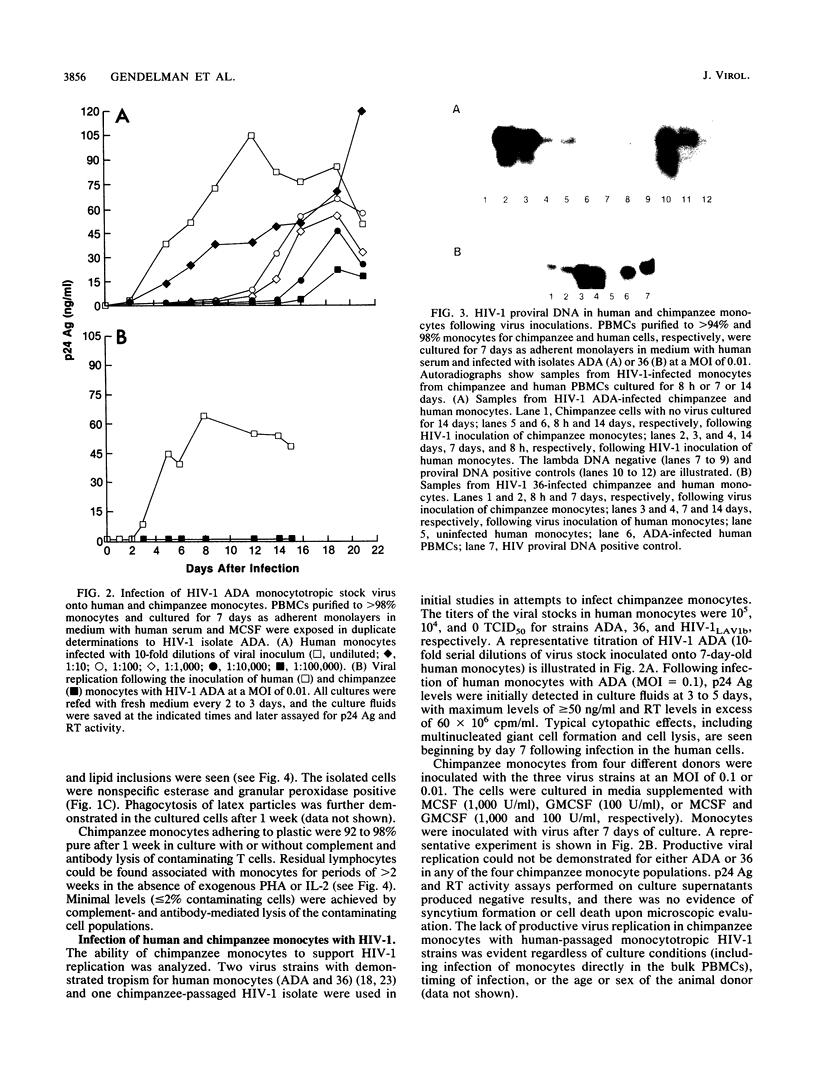
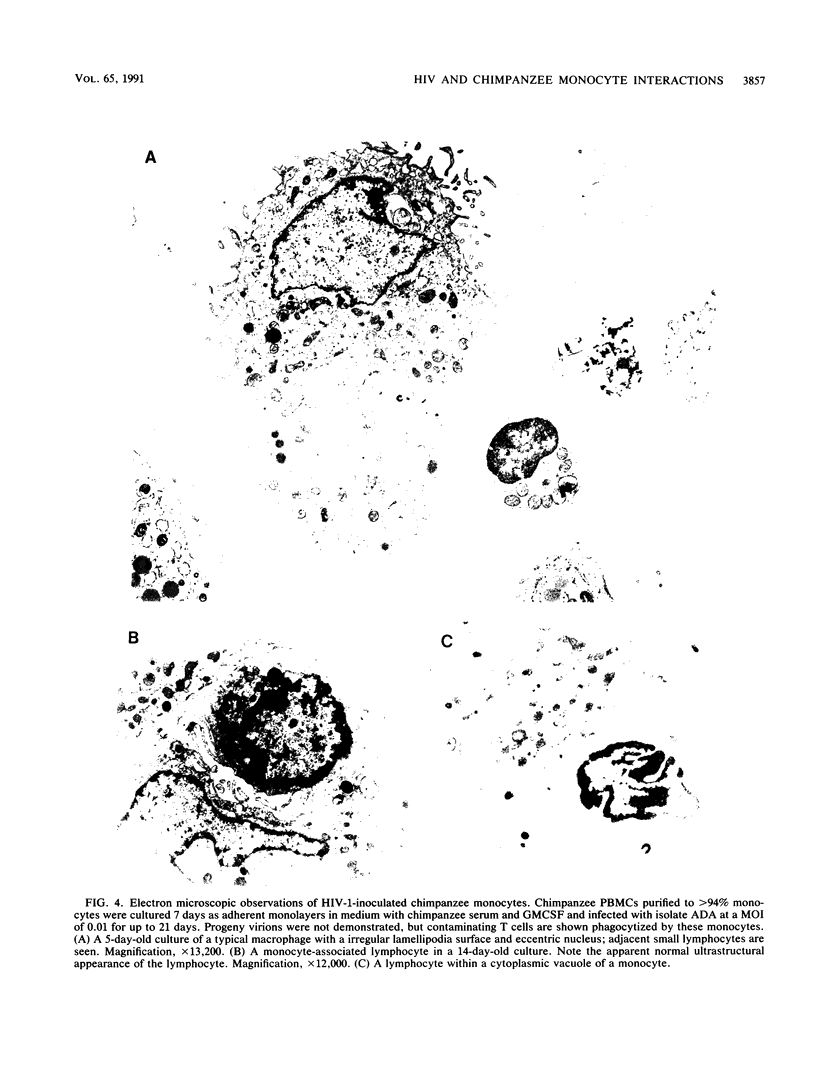
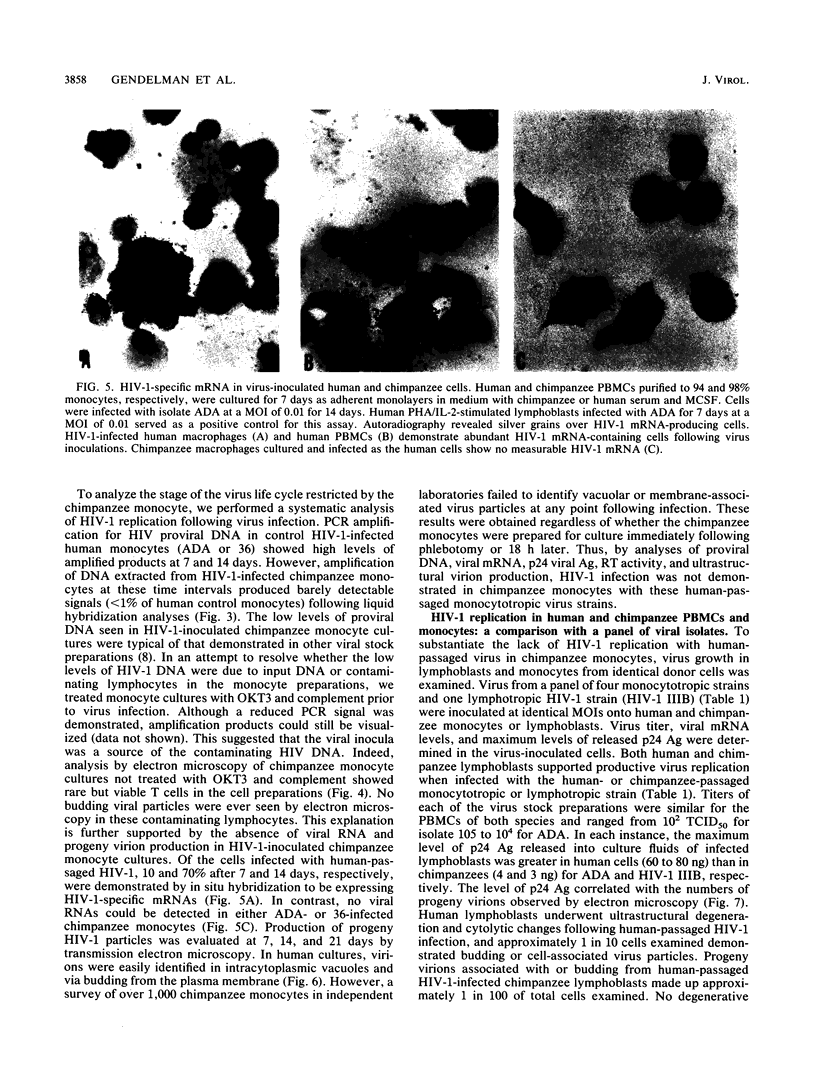
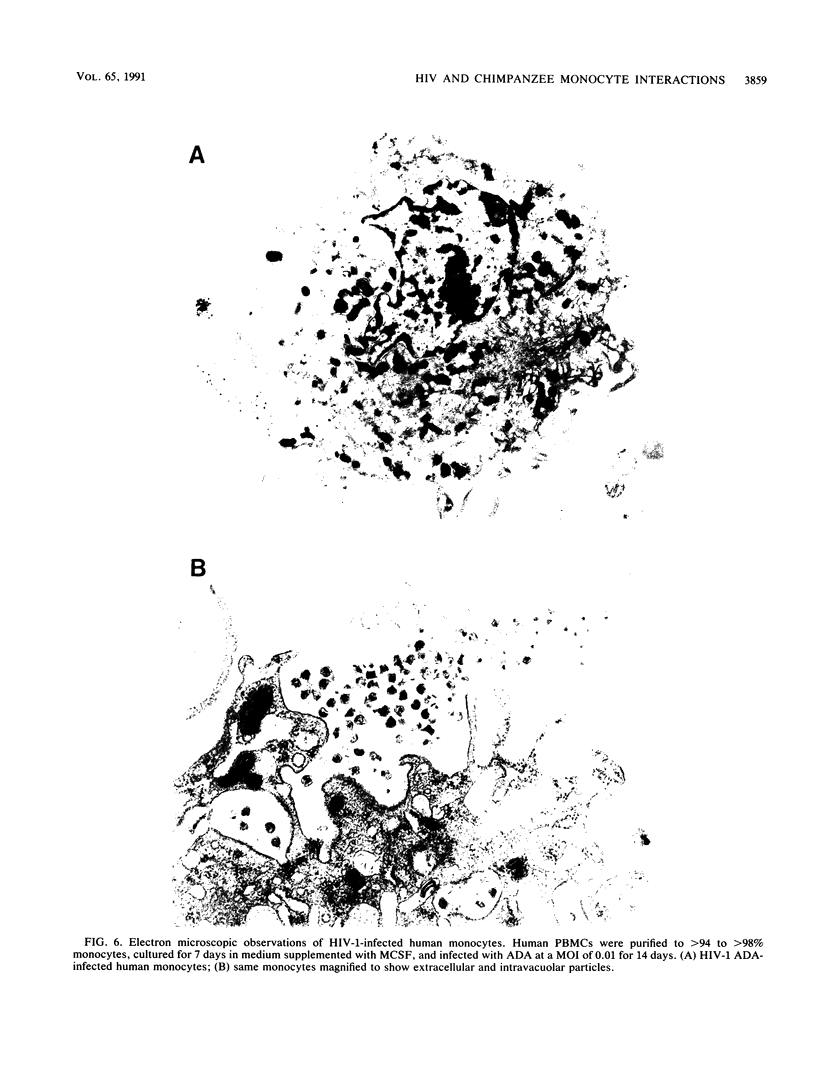
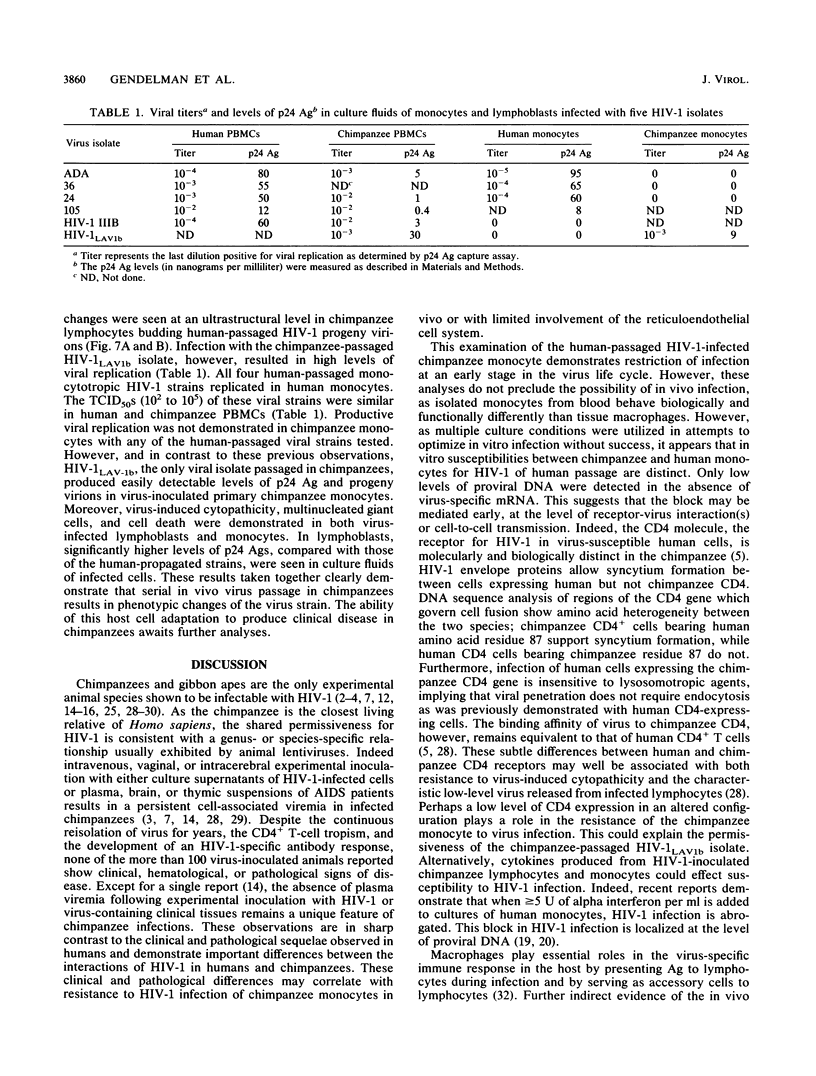
Studies of lentivirus infection in ruminants, nonhuman primates, and humans suggest that virus infection of macrophages plays a central role in the disease process. To investigate whether human immunodeficiency virus type 1 (HIV-1) can infect chimpanzee macrophages, we recovered monocytes from peripheral blood mononuclear cells of HIV-1-negative animals and inoculated these and control human monocytes with a panel of four human-passaged monocytotropic virus strains and one chimpanzee-passaged isolate. HIV-1 infected human monocytes synthesized proviral DNA, viral mRNA, p24 antigen, and progeny virions. In contrast, except for the chimpanzee-passaged HIV-1 isolate, chimpanzee monocytes failed to support HIV-1 replication when cultured under both identical and a variety of other conditions. Proviral DNA was demonstrated only at background levels in these cell cultures by polymerase chain reaction for gag- and env-related sequences. Interestingly, the chimpanzee-passaged HIV-1 isolate did not replicate in human monocytes; viral p24 antigens and progeny virions were not detected. The same monocytotropic panel of HIV-1 strains replicated in both human and chimpanzee CD4+ T lymphoblasts treated with phytohemagglutinin and interleukin-2. The failure of HIV-1 to infect chimpanzee monocytes, which can be overcome by serial in vivo viral passage, occurs through a block early in the viral life cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott M. A., Poiesz B. J., Byrne B. C., Kwok S., Sninsky J. J., Ehrlich G. D. Enzymatic gene amplification: qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J Infect Dis. 1988 Dec;158(6):1158–1169. doi: 10.1093/infdis/158.6.1158. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Eichberg J. W., Masur H., Saxinger W. C., Gallo R., Macher A. M., Lane H. C., Fauci A. S. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984 Nov 2;226(4674):549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- Arthur L. O., Bess J. W., Jr, Waters D. J., Pyle S. W., Kelliher J. C., Nara P. L., Krohn K., Robey W. G., Langlois A. J., Gallo R. C. Challenge of chimpanzees (Pan troglodytes) immunized with human immunodeficiency virus envelope glycoprotein gp120. J Virol. 1989 Dec;63(12):5046–5053. doi: 10.1128/jvi.63.12.5046-5053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur L. O., Pyle S. W., Nara P. L., Bess J. W., Jr, Gonda M. A., Kelliher J. C., Gilden R. V., Robey W. G., Bolognesi D. P., Gallo R. C. Serological responses in chimpanzees inoculated with human immunodeficiency virus glycoprotein (gp120) subunit vaccine. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8583–8587. doi: 10.1073/pnas.84.23.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerini D., Seed B. A CD4 domain important for HIV-mediated syncytium formation lies outside the virus binding site. Cell. 1990 Mar 9;60(5):747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- Collman R., Hassan N. F., Walker R., Godfrey B., Cutilli J., Hastings J. C., Friedman H., Douglas S. D., Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989 Oct 1;170(4):1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. D., Glaser J. B., LaVigne K., Quan D., Mildvan D., Sninsky J. J., Kwok S., Papsidero L., Poiesz B. J. Prevalence of human T-cell leukemia/lymphoma virus (HTLV) type II infection among high-risk individuals: type-specific identification of HTLVs by polymerase chain reaction. Blood. 1989 Oct;74(5):1658–1664. [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Feorino P. M., Broderson J. R., McClure H. M., Getchell J. P., McGrath C. R., Swenson B., McDougal J. S., Palmer E. L., Harrison A. K. Infection of chimpanzees with lymphadenopathy-associated virus. Lancet. 1984 Dec 1;2(8414):1276–1277. doi: 10.1016/s0140-6736(84)92824-1. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Swenson R. B., McGrath C. R., Brodie A., Getchell J. P., Jensen F. C., Anderson D. C., Broderson J. R., Francis D. P. Persistent infection of chimpanzees with human T-lymphotropic virus type III/lymphadenopathy-associated virus: a potential model for acquired immunodeficiency syndrome. J Virol. 1986 Apr;58(1):116–124. doi: 10.1128/jvi.58.1.116-124.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek D. C., Amyx H. L., Gibbs C. J., Jr, Asher D. M., Rodgers-Johnson P., Epstein L. G., Sarin P. S., Gallo R. C., Maluish A., Arthur L. O. Infection of chimpanzees by human T-lymphotropic retroviruses in brain and other tissues from AIDS patients. Lancet. 1985 Jan 5;1(8419):55–56. doi: 10.1016/s0140-6736(85)91011-6. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C., Amyx H. L., Gibbs C. J., Jr, Asher D. M., Yanagihara R. T., Rodgers-Johnson P., Brown P. W., Sarin P. S., Gallo R. C., Jr, Maluish A. Transmission experiments with human T-lymphotropic retroviruses and human AIDS tissue. Lancet. 1984 Jun 23;1(8391):1415–1416. doi: 10.1016/s0140-6736(84)91916-0. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Husayni H., Turpin J. A., Skillman D., Kalter D. C., Orenstein J. M., Hoover D. L., Meltzer M. S. Macrophage-HIV interaction: viral isolation and target cell tropism. AIDS. 1990 Mar;4(3):221–228. [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Turpin J., Kalter D. C., Hansen B., Orenstein J. M., Dieffenbach C. W., Friedman R. M., Meltzer M. S. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol. 1990 Oct 15;145(8):2669–2676. [PubMed] [Google Scholar]
- Gendelman H. E., Friedman R. M., Joe S., Baca L. M., Turpin J. A., Dveksler G., Meltzer M. S., Dieffenbach C. A selective defect of interferon alpha production in human immunodeficiency virus-infected monocytes. J Exp Med. 1990 Nov 1;172(5):1433–1442. doi: 10.1084/jem.172.5.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Baca L. M., Weiser B., Burger H., Kalter D. C., Meltzer M. S. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989 Aug;3(8):475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., Markham P. D., Ranki A., Earl P., Moss B., Dorner F., Gallo R. C., Krohn K. J. Cell-mediated immune response toward viral envelope and core antigens in gibbon apes (Hylobates lar) chronically infected with human immunodeficiency virus-1. J Immunol. 1988 Oct 1;141(7):2467–2473. [PubMed] [Google Scholar]
- Nakanuma Y., Liew C. T., Peters R. L., Govindarajan S. Pathologic features of the liver in acquired immune deficiency syndrome (AIDS). Liver. 1986 Jun;6(3):158–166. doi: 10.1111/j.1600-0676.1986.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Smit L., Dunlop N., Hatch W., Merges M., Waters D., Kelliher J., Gallo R. C., Fischinger P. J., Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990 Aug;64(8):3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P., Hatch W., Kessler J., Kelliher J., Carter S. The biology of human immunodeficiency virus-1 IIIB infection in the chimpanzee: in vivo and in vitro correlations. J Med Primatol. 1989;18(3-4):343–355. [PubMed] [Google Scholar]
- Narayan O., Clements J. E. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989 Jul;70(Pt 7):1617–1639. doi: 10.1099/0022-1317-70-7-1617. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Lequarre A. S., Casey J. W., Lahn S., Stephens R. M., Edwards J. Viral DNA in horses infected with equine infectious anemia virus. J Virol. 1989 Dec;63(12):5194–5200. doi: 10.1128/jvi.63.12.5194-5200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Gendrault J. L., Schweitzer C., Steffan A. M., Beyer C., Royer C., Jaeck D., Pasquali J. L., Kirn A., Aubertin A. M. Permissivity of primary cultures of human Kupffer cells for HIV-1. AIDS Res Hum Retroviruses. 1990 Aug;6(8):987–991. doi: 10.1089/aid.1990.6.987. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., McCutchan J. A., Venable J. C., Nelson J. A., Wiley C. A. T-cell-induced expression of human immunodeficiency virus in macrophages. J Virol. 1990 Jul;64(7):3280–3288. doi: 10.1128/jvi.64.7.3280-3288.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Ringler D. J., Fultz P. N., MacKey J. J., Boyson J. E., Levine C. G., Letvin N. L. A chimpanzee-passaged human immunodeficiency virus isolate is cytopathic for chimpanzee cells but does not induce disease. J Virol. 1991 Jun;65(6):3344–3348. doi: 10.1128/jvi.65.6.3344-3348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Eichberg J. W., Moran P. A., McClure J., Sridhar P., Hu S. L. Proliferative and cytotoxic T cells to AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virus expressing AIDS virus envelope glycoproteins. J Immunol. 1987 Aug 15;139(4):988–990. [PubMed] [Google Scholar]