Abstract
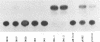
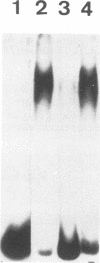
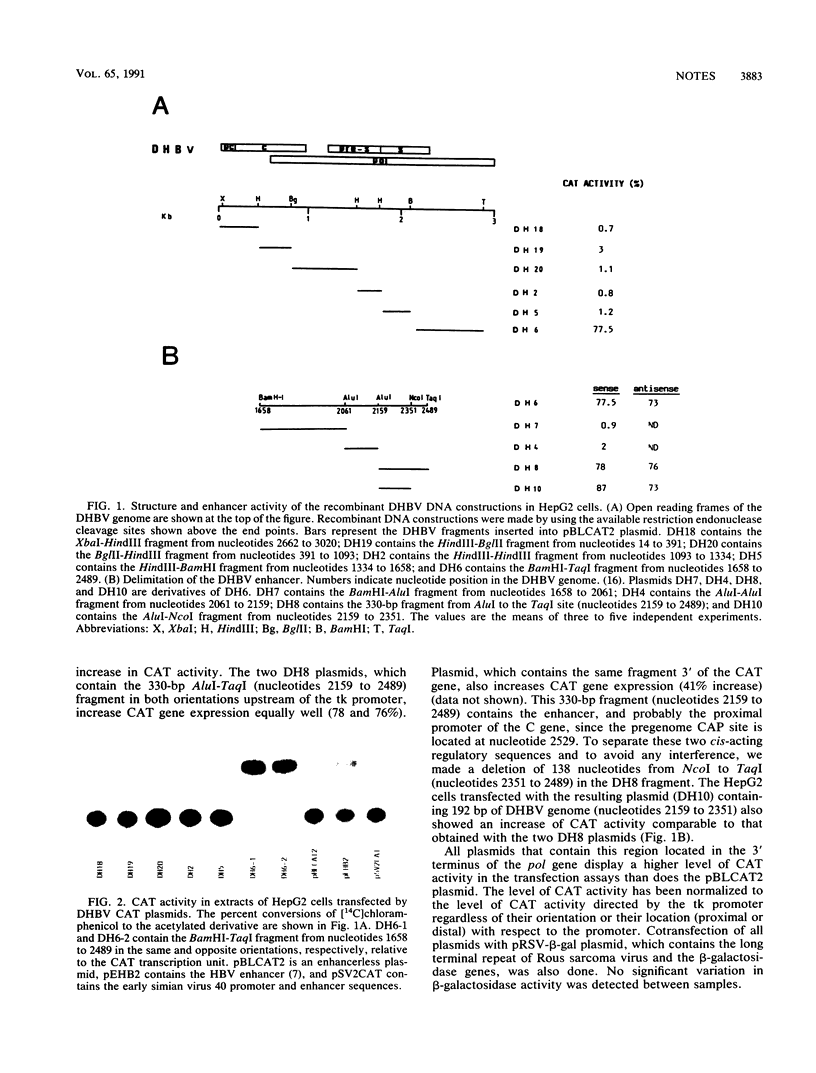
The genome of the duck hepatitis B virus (DHBV) contains an enhancer element. This sequence, of 192 bp, is located in the 3'-terminal coding region of the DNA polymerase gene (nucleotides 2159 to 2351), upstream from the pregenomic RNA start site. This enhancer potentiates a marked increased activity from the heterologous thymidine kinase promoter in an orientation-independent manner and at a proximal, as well as a distal, location. The DHBV enhancer activates transcription in a relatively cell-type-independent manner. Sequence homologies with the nuclear factor EF-C binding site are located in the DHBV enhancer. By using the HepG2 nuclear extracts and the DHBV enhancer as probes, a complex was observed in mobility shift assays.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aufiero B., Schneider R. J. The hepatitis B virus X-gene product trans-activates both RNA polymerase II and III promoters. EMBO J. 1990 Feb;9(2):497–504. doi: 10.1002/j.1460-2075.1990.tb08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenschlager R., Junker-Niepmann M., Schaller H. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J Virol. 1990 Nov;64(11):5324–5332. doi: 10.1128/jvi.64.11.5324-5332.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. J., Ganem D., Varmus H. E. Mechanism of translation of the hepadnaviral polymerase (P) gene. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5158–5162. doi: 10.1073/pnas.87.13.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgrove R., Simon G., Ganem D. Transcriptional activation of homologous and heterologous genes by the hepatitis B virus X gene product in cells permissive for viral replication. J Virol. 1989 Sep;63(9):4019–4026. doi: 10.1128/jvi.63.9.4019-4026.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikstein R., Faktor O., Ben-Levy R., Shaul Y. Functional organization of the hepatitis B virus enhancer. Mol Cell Biol. 1990 Jul;10(7):3683–3689. doi: 10.1128/mcb.10.7.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfassi E. Broad specificity of the hepatitis B enhancer function. Virology. 1987 Sep;160(1):259–262. doi: 10.1016/0042-6822(87)90069-9. [DOI] [PubMed] [Google Scholar]
- Galle P. R., Schlicht H. J., Fischer M., Schaller H. Production of infectious duck hepatitis B virus in a human hepatoma cell line. J Virol. 1988 May;62(5):1736–1740. doi: 10.1128/jvi.62.5.1736-1740.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., England J. M., Flores L., Egan J., Newbold J., Mason W. S. Individual cells in tissues of DHBV-infected ducks express antigens crossreactive with those on virus surface antigen particles and immature viral cores. Virology. 1984 Sep;137(2):408–413. doi: 10.1016/0042-6822(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Junker-Niepmann M., Bartenschlager R., Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990 Oct;9(10):3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levrero M., Balsano C., Natoli G., Avantaggiati M. L., Elfassi E. Hepatitis B virus X protein transactivates the long terminal repeats of human immunodeficiency virus types 1 and 2. J Virol. 1990 Jun;64(6):3082–3086. doi: 10.1128/jvi.64.6.3082-3086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Diffley J. F., Bruder J. T., Stillman B., Levine A. J., Hearing P. Interaction of a nuclear factor with the polyomavirus enhancer region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8550–8554. doi: 10.1073/pnas.83.22.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Scheirle G., Hearing P. Binding of nuclear factor EF-C to a functional domain of the hepatitis B virus enhancer region. Mol Cell Biol. 1989 Jul;9(7):2787–2797. doi: 10.1128/mcb.9.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei D. Q., Shih C. H. Transcriptional activation and repression by cellular DNA-binding protein C/EBP. J Virol. 1990 Apr;64(4):1517–1522. doi: 10.1128/jvi.64.4.1517-1522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwill G., Tucker W., Schaller H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol. 1990 Feb;64(2):613–620. doi: 10.1128/jvi.64.2.613-620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Tognoni A., Cattaneo R., Serfling E., Schaffner W. A novel expression selection approach allows precise mapping of the hepatitis B virus enhancer. Nucleic Acids Res. 1985 Oct 25;13(20):7457–7472. doi: 10.1093/nar/13.20.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Levinson A. D. Properties of the human hepatitis B virus enhancer: position effects and cell-type nonspecificity. J Virol. 1988 Apr;62(4):1305–1313. doi: 10.1128/jvi.62.4.1305-1313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]