Abstract
Although the lateral nucleus of the amygdala (LA) is essential for conditioned auditory fear memory, an emerging theme is that plasticity in multiple brain regions contributes to fear memory formation. The LA receives direct projections from the auditory thalamus, specifically the medial division of the medial geniculate nucleus (MGm) and adjacent posterior intralaminar nucleus (PIN). While traditionally viewed as a simple relay structure, mounting evidence implicates the thalamus in diverse cognitive processes. We investigated the role of plasticity in the MGm/PIN in auditory fear memory. First we found that auditory fear conditioning (but not control manipulations) increased the levels of activated CREB in both the MGm and PIN. Next, using viral vectors, we showed that exogenously increasing CREB in this region specifically enhanced formation of an auditory conditioned fear memory without affecting expression of an auditory fear memory, formation of a contextual fear memory, or basic auditory processing. Interestingly, mice with increased CREB levels in the MGm/PIN also showed broad auditory fear generalization (in contrast to control mice, they exhibited fear responses to tones of other frequencies). Together, these results implicate CREB-mediated plasticity in the MGm/PIN in both the formation and generalization of conditioned auditory fear memory. Not only do these findings refine our knowledge of the circuitry underlying fear memory but they also provide novel insights into the neural substrates that govern the degree to which acquired fear of a tone generalizes to other tones.
Auditory fear conditioning is commonly used to probe the neural substrates of memory. In this task, an initially neutral tone (conditioned stimulus [CS]) is paired with shock (unconditioned stimulus [US]) (Kapp et al. 1979; LeDoux 2000; Davis and Whalen 2001; Fanselow and Gale 2003). Upon subsequent tone presentation, animals exhibit conditioned fear responses, including freezing (Anagnostaras et al. 2000; LeDoux 2000; Fanselow and Gale 2003). Plasticity in the amygdala, particularly the lateral nucleus (LA), is critical for long-term memory for conditioned auditory fear (Davis 1992; Fanselow and LeDoux 1999; Maren and Quirk 2004; Dityatev and Bolshakov 2005; but see Cahill et al. 1999). Accordingly, disrupting plasticity in the LA by locally perturbing transcription or translation impairs long-term memory for auditory fear conditioning (Bailey et al. 1999; Schafe and LeDoux 2000; Maren et al. 2003). On the other hand, increasing the function of the transcription factor CREB (cAMP/Ca2+ responsive element binding protein) in the LA enhances memory for conditioned fear (Josselyn et al. 2001; Wallace et al. 2004; Han et al. 2007). The requirement of LA plasticity, however, does not exclude important contributions from additional regions.
The LA receives direct projections from auditory thalamus (medial geniculate nucleus [MGN]), specifically the medial division of MGN and adjacent posterior intralaminar nucleus (MGm/PIN) (LeDoux et al. 1990; Doron and Ledoux 2000; Radley et al. 2007). Although traditionally viewed as a relay structure, mounting evidence links the thalamus to diverse cognitive processes (Crick 1984; Komura et al. 2001; Kassubek et al. 2005; Minamimoto et al. 2005; McAlonan et al. 2006). Indeed several findings implicate the MGm/PIN in conditioned auditory fear memory. First, this region receives both auditory and somatosensory inputs (Bordi and LeDoux 1994b; Simone et al. 2004), suggesting that it is a site of CS/US convergence. Second, auditory fear training induces associative neuronal activity in the MGm (McEchron et al. 1996; Maren et al. 2001) and high-frequency stimulation induces long-term potentiation (LTP) in the MGm (Gerren and Weinberger 1983).
Although these studies are consistent with the notion that plasticity in the MGm/PIN plays a role in conditioned auditory fear memory, experiments designed to explicitly test this prediction are inconclusive. While several labs showed that intra-thalamic infusions of RNA synthesis inhibitors block memory for auditory fear (Apergis-Schoute et al. 2005; Parsons et al. 2006), similar infusions of protein synthesis inhibitors were reported to block (Parsons et al. 2006) or produce no effect (Maren et al. 2003; Apergis-Schoute et al. 2005; Parsons et al. 2006) on memory for conditioned auditory fear. In addition, because these intra-thalamic drug infusions may diffuse to adjacent brain regions, the precise areas that mediate potential effects are unclear. Finally, the functional significance of MGm/PIN plasticity in fear conditioning is largely unknown.
As an alternative to examining the effects of disrupting plasticity, we used a gain-of-function approach. We found that auditory fear conditioning specifically increased the activated levels of the transcription factor CREB (pCREB) in both the MGm and PIN. Next, we used viral vectors to exogenously increase CREB levels in this region. Increasing CREB specifically in neuronal nuclei in the MGm/PIN enhanced the formation of auditory conditioned fear memory. Similarly, increasing CREB had no effect on the expression of auditory fear memory or general auditory processing. We also found that increasing CREB in the MGm/PIN enhanced the generalization of fear responses to tones of other frequencies. This fear overgeneralization was modality (tone)-specific and did not extend to contextual fear conditioning. Together, these data implicate CREB-mediated plasticity in the MGm/PIN in the formation and generalization of conditioned auditory fear memory.
Results
Auditory fear conditioning activates CREB in the MGm/PIN
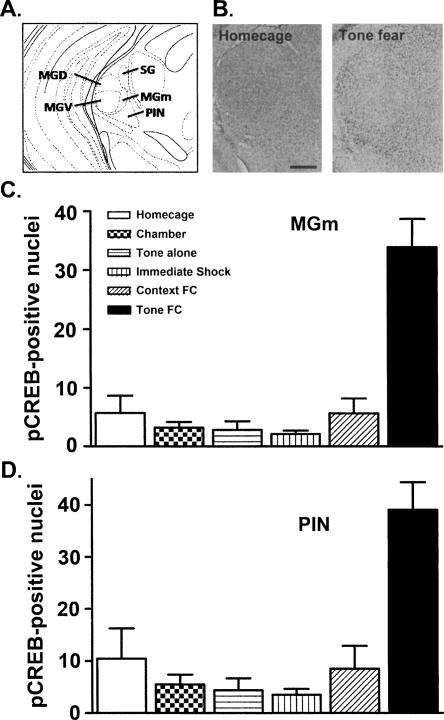
Previous studies generally indicate that CREB plays an important role in the plasticity underlying long-term memory in both invertebrates (Dash et al. 1990; Yin et al. 1995; Wagatsuma et al. 2006) and vertebrates (Bourtchuladze et al. 1994; Guzowski and McGaugh 1997; Lamprecht et al. 1997; Graves et al. 2002; Kida et al. 2002; Pittenger et al. 2002; Josselyn et al. 2004; but see Balschun et al. 2003). The ultimate products of CREB-mediated transcription are thought to contribute to the synaptic remodeling mediating long-term memory (Sheng and Greenberg 1990; Frank and Greenberg 1994; Desmedt et al. 2003). Because CREB-mediated transcription can be initiated by phosphorylation of the Ser 133 residue (Gonzalez and Montminy 1989), CREB activation (CREB that is phosphorylated at Ser 133, pCREB) is commonly used as an immunocytochemical marker of brain regions undergoing plasticity during learning (Impey et al. 1998; Desmedt et al. 2003). Accordingly, previous studies show that training that induces long-term memory for conditioned fear is associated with an increase in the levels of activated CREB in the basolateral/lateral amygdala of mice (Stanciu et al. 2001; Han et al. 2007). Here, we examined whether similar auditory fear training also increases CREB activation in the MGm and/or PIN (Fig. 1A). Figure 1B shows that pCREB levels in both the MGm and PIN are indeed increased following auditory fear conditioning compared to homecage controls.
Figure 1.
Training that induces long-term memory for auditory fear conditioning increases the levels of activated CREB (pCREB) in both the MGm and PIN. (A) Location of the MGm (medial division of the medial geniculate nucleus) and adjacent PIN (posterior intralaminar nucleus) (thick outline) in the thalamus. Adapted from Paxinos and Franklin (2003). (B) Auditory fear conditioning (Tone fear, right) increased levels of activated CREB (pCREB) in the MGm and PIN compared to a control (Homecage, left) condition (scale bar = 100 μm). (C,D) Quantification of pCREB levels in the MGm (C) and PIN (D) following auditory fear conditioning (Tone+Shock) and several control training conditions (Homecage, Chamber, Tone alone, Immediate shock, Context fear conditioning). The increase in pCREB levels in both the MGm and PIN is specific to training that produces auditory fear memory (Tone FC). Number of pCREB-positive nuclei per 100 × 100 μm field is shown.
Activation of CREB in the MGm and PIN is specific to associative tone-shock pairing
To determine whether the increase in pCREB levels in the MGm and PIN was specifically induced following auditory fear conditioning (tone-shock pairing), rather than nonassociative aspects of the procedure (such as auditory stimulation, shock, or placement in the chamber), we similarly examined pCREB levels following several control training conditions (tone alone, context fear conditioning, immediate shock training, chamber alone, and homecage).
Figure 1C,D shows that auditory fear conditioning (Tone+Shock), but not any of the control training manipulations, increased pCREB levels in both the MGm and PIN, respectively. Separate ANOVAs performed on the number of pCREB positive nuclei in the MGm and PIN using Treatment (Tone+Shock, Tone alone, Chamber+Shock, Immediate Shock, Chamber alone, and Homecage) as a between-group factor revealed a significant effect in both the MGm (F(5,20) = 20.92; P < 0.001) and PIN (F(5,20) = 12.67; P < 0.001). Post-hoc Bonferroni comparisons showed that auditory fear conditioning increased pCREB levels relative to all control conditions (which did not differ from each other). Interestingly, pCREB levels in the MGm and PIN were similar following auditory fear training and control manipulations (F(5,20) = 1.11; P > 0.05, no significant interaction between Brain Region and Treatment). Together, these data indicate that CREB is normally activated in both the MGm and PIN following auditory fear conditioning and that this activation is not produced by nonassociative factors (such as auditory stimulation, shock, chamber placement, or stress).
To further investigate the role of CREB-mediated plasticity in the MGm/PIN region in auditory fear conditioning, we exogenously increased CREB levels and examined the effects on auditory fear memory. Because plasticity in the LA has been shown by many research groups to be critical for conditioned auditory fear memory, as a confirmatory step, we first examined the effects of increasing CREB levels in the LA on auditory fear memory.
Effects of shock intensity on auditory fear conditioning
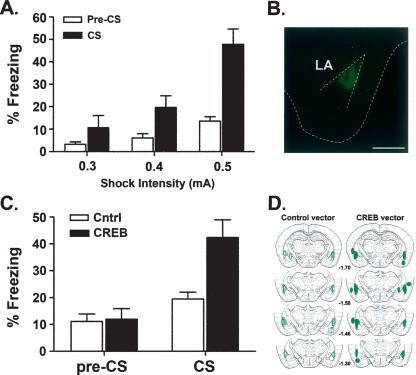
To examine the effects of increasing CREB function in the LA or MGm/PIN on auditory fear conditioning memory, we first determined the training conditions that induced suboptimal memory. In this way, a potential enhancement of memory, as indicated by an increase in freezing levels, could be observed in subsequent experiments free from the potential masking by ceiling effects. We trained unoperated control mice with one tone-shock pairing but varied the intensity of the shock (0.3-, 0.4-, and 0.5-mA shock; n = 7, 10, and 8, respectively). Mice were tested 24 h later and the percentage of time spent freezing before (pre-CS freezing) and during the tone (CS freezing) was assessed. Figure 2A shows the mean (±SEM) percent time mice spent freezing during the test session, both before (pre-CS) and during (CS) the tone. As can be seen from this figure, the freezing levels did not differ between the groups before the CS was replayed. However, mice that received more intense shocks during training froze at higher levels to the CS. An ANOVA with between-group factor Shock Intensity (0.3, 0.4, and 0.5 mA) and within-group factor Time (Pre-CS and CS) showed a significant effect of Shock Intensity (F(2,22) = 12.12; P < 0.001), Time (F(1,22) = 35.11; P < 0.001), and Shock Intensity × Time interaction (F(2,22) = 6.54; P < 0.001). Post-hoc Bonferroni tests revealed that the pre-CS freezing levels did not differ between the groups (P > 0.05), but freezing levels during the CS were greater in the 0.5-mA group (P < 0.05) than in the 0.4- and 0.3-mA groups (which did not differ, P > 0.05). Therefore, in subsequent experiments, we trained mice with a 0.4-mA shock.
Figure 2.
Increasing CREB levels in the LA of mice enhances memory for auditory conditioned fear. (A) Determining the parameters for inducing low levels of auditory conditioned fear in mice. Unoperated control mice were trained with one tone-shock pairing with shock intensities of 0.3, 0.4, or 0.5 mA and tested 24 h later. The mean (±SEM) percent time mice spent freezing in the test session before (Pre-CS) and during (CS) the one-minute tone is shown. Mice trained with a 0.4-mA shock showed nonceiling levels of auditory fear conditioning when tested 24 h following training. (B) Robust, localized transgene expression of GFP following infusion of vectors into the LA (scale bar = 100 μm). (C) Mice infused with the CREB vector into the LA showed enhanced memory for auditory fear conditioning. The mean (±SEM) percent time mice infused with Control (Cntrl) or CREB vector in the LA spent freezing in the test session before (Pre-CS) and during (CS) the tone. (D) Areas of highest transgene expression following infusion of CREB or Control vector.
Increasing CREB in the LA enhances memory for auditory conditioned fear
Previously, we and others showed that increasing CREB levels in the LA, via viral-mediated gene transfer, enhanced long-term memory for fear conditioning in rats (Josselyn et al. 2001; Wallace et al. 2004) and mice (Han et al. 2007). We used a replication-defective herpes simplex virus (HSV) for these studies because, unlike many other viruses, HSV is naturally neurotropic (Fink et al. 1996). Previous studies established that infusion of this CREB vector increased both CREB levels and function (CRE-mediated transcription) (Barrot et al. 2002; Olson et al. 2005). In the present experiment, mice received intra-LA infusions of CREB or Control vector 3 d prior to auditory fear conditioning. To examine whether increasing CREB function enhanced memory, we used a minimal training protocol (0.4-mA shock) that induces only weak long-term memory.
Histology
Figure 2B shows an example of the distribution of GFP expression following vector infusion into the LA. Consistent with previous results from several labs, minimal tissue damage in, and around, the infusion site was observed (Carlezon and Neve 2003; Neve et al. 2005). Figure 2D shows the placement and spread of vector infusions, as determined by the highest level of GFP expression, for mice infused with the CREB and Control vectors. Only those mice with robust transgene expression bilaterally in the LA were included in subsequent statistical analysis (CREB vector n = 9, Control vector n = 10). Of the 15 mice infused with the CREB vector, one was off-target bilaterally and five were off-target unilaterally. In the Control vector group, a total of 13 mice were infused, three of which had unilateral off-target placements.
Behavior
As can be seen from Figure 2C, infusing the CREB vector into the LA of mice specifically increased the levels of freezing to the tone. This observation is supported by the results of an ANOVA (Vector [CREB vs. Control] × Time [pre-CS vs. CS]) in which significant effects of Vector (F(1,17) = 5.37; P < 0.05), Time (F(1,17) = 43.71; P < 0.001), and a Vector-by-Time interaction (F(1,17) = 14.20; P < 0.001) were found. Mice infused with the CREB vector showed higher levels of freezing during the tone than mice infused with the Control vector (P < 0.001), but levels of freezing during the pre-CS period were not significantly different (P > 0.05), as determined by Bonferroni post-hoc comparisons of the significant interaction. Therefore, consistent with previous findings in rats (Josselyn et al. 2001; Wallace et al. 2004), mice (Han et al. 2007), and hamsters (Jasnow et al. 2005), we found that increasing CREB in the LA enhances memory.
Increasing CREB in the MGm/PIN specifically enhances memory for auditory conditioned fear
The above results show that increasing CREB in the LA enhanced conditioned auditory fear memory. We used a similar strategy to examine the effects of increasing CREB levels in the MGm/PIN. Mice were fear-conditioned using the same minimal training parameters.
Histology
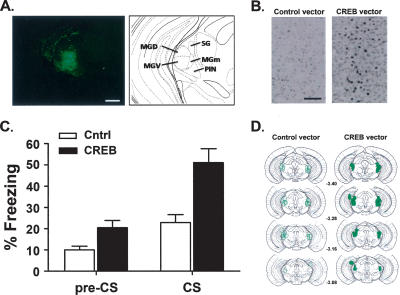
Figure 3A shows an example of the expression pattern of GFP following infusion of vectors aimed at the MGm/PIN region. Consistent with previous results, the extent of GFP-positive (infected) neurons was restricted to an area roughly 0.5 mm in diameter around the tip of the injection site (Carlezon and Neve 2003; Neve et al. 2005). Nonetheless, GFP-positive neurons were observed both in the targeted region (MGm/PIN) and in neighboring regions of the auditory thalamus, including the ventral and dorsal divisions of the MGN (MGv, MGd) and suprageniculate nucleus (SG). Figure 3B shows that infusing the CREB (but not Control) vector into the MGm/PIN region indeed increases CREB levels, as assessed by immunohistochemistry for CREB. This finding is consistent with previous studies showing that infusion of this CREB vector increases both CREB levels and function in several brain regions (Barrot et al. 2002; Olson et al. 2005).
Figure 3.
Increasing CREB levels in the MGm/PIN region of mice enhances memory for auditory conditioned fear. (A) Robust localized transgene expression following infusion of vectors into the MGm/PIN region. Scale bar = 100 μm. (B) Infusing the CREB vector, but not the Control vector, into this region enhances CREB levels in the MGm/PIN. This robust increase in CREB immunostaining is likely underestimated in this preparation because the immunocytochemical conditions were adjusted so as to be minimally sensitive to the background (endogenous) levels of CREB. Scale bar = 50 μm. (C) Mice infused with the CREB vector into the MGm/PIN showed enhanced memory for auditory fear conditioning. The mean (±SEM) percent time mice infused with Control (Cntrl) or CREB vector in the MGm/PIN spent freezing in the test session before (Pre-CS) and during (CS) the tone. (D) Areas of highest transgene expression following infusion of CREB or Cntrl vector aimed at the MGm/PIN.
Figure 3D shows the placement and extent of infection for mice infused with the CREB or Control vector. The number of mice injected with the CREB or Control vector was 36 and 22, respectively. In the CREB vector group, 14 mice showed robust bilateral expression of GFP in the MGm/PIN. Three mice were excluded from subsequent data analysis because of improper histology, eight mice showed unilateral infection in the MGm/PIN, and 11 mice were infused off-target bilaterally (typically in the MGd, MGv, SG, and/or dentate gyrus). In the Control vector group, 11 mice were included in the data analysis, four mice showed only unilateral expression in the target region, and seven mice showed expression that was bilaterally off-target.
Behavior
Figure 3C shows that infusing the CREB vector into the MGm/PIN specifically increased freezing to the CS. This observation is supported by the results of a Vector-by-Time ANOVA in which significant effects of Vector (F(1,23) = 12.32; P < 0.001), Time (F(1,23) = 41.06; P < 0.001), and a Vector-by-Time interaction (F(1,23) = 6.80; P < 0.001) were found. Bonferroni post-hoc comparisons of the significant interaction revealed that freezing levels did not differ between the groups during the pre-CS period (P > 0.05), but that mice infused with the CREB vector froze more during the tone CS than mice infused with the Control vector (P < 0.001). The lack of difference between pre-CS freezing levels indicates that increasing CREB in the MGm/PIN does not increase fear or anxiety-like behavior nonspecifically. It is also important to note that infusion of the Control vector into the MGm/PIN did not appear to alter conditioned auditory fear memory. Unoperated control mice and mice infused with the Control vector showed similar levels of freezing to the tone CS (unoperated mice trained using a 0.4-mA intensity shock, CS freezing = 19.67 ± 5.18, Fig. 2A; mice with Control vector injected into the MGm/PIN, CS freezing = 22.87 ± 3.77, Fig. 3C). Together, these findings indicate that increasing CREB in the MGm/PIN specifically enhances memory for conditioned auditory fear.
Correlating the location and number of CREB-infected neurons with memory
Conditioned auditory fear memory correlates with the number of MGm/PIN neurons infected with the CREB vector
The use of HSV vectors allows precise manipulations of genes of interest in specific target regions. Because CREB is fused with GFP in our vector, it is possible to visualize the specific location of infected neuronal nuclei. Although our infusions targeted neurons with cell bodies in the MGm/PIN, neurons in neighboring regions were also infected. Indeed, a unique pattern of vector infection was observed in each mouse. We took advantage of this to determine the precise neural region supporting the observed memory enhancement. Specifically, we examined whether the number of CREB-infected neurons in the MGm/PIN or adjacent auditory thalamic regions (grouped as MGd/MGv/SG) correlated with the level of auditory fear memory. We counted the number of infected neurons (GFP-positive nuclei) in the MGm/PIN and MGd/MGv/SG regions of the auditory thalamus in mice categorized as having accurate bilateral or unilateral placements. Twenty-two mice were included in the MGm/PIN–CREB vector group (14 with bilateral placements, eight with unilateral placements).
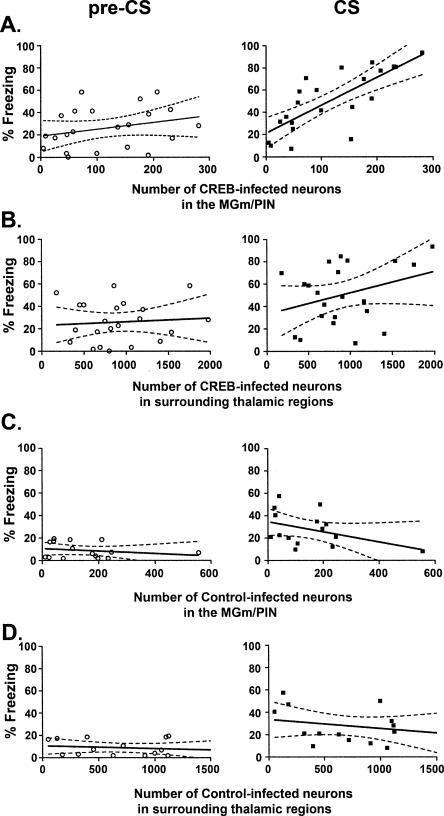
As can be seen from Figure 4A, there was a high correlation between the number of CREB-infected neurons in the MGm/PIN and the level of freezing to the tone CS. The best fit line for this correlation was y = 0.25x + 21.13. Regression analysis revealed a significant correlation between the number of CREB-infected neurons and tone freezing levels (F(1,20) = 28.44; P < 0.001; R2 = 0.59). In contrast, the number of CREB-infected neurons in the MGm/PIN did not correlate with baseline (pre-CS) freezing levels (F(1,20) = 4.34; P > 0.05; R2 = 0.18; best fit line, y = 0.090x + 16.11). Therefore, the higher the number of MGm/PIN nuclei with the CREB vector, the greater the auditory conditioned fear memory. This increase in freezing was specific to the tone (not to baseline pre-CS freezing).
Figure 4.
Auditory conditioned fear memory correlates with the number of neuronal nuclei infected with the CREB vector in the MGm/PIN region only, showing the behavioral and regional specificity of the CREB-induced memory enhancement. (A) The number CREB-infected neurons in the MGm/PIN positively correlated with the mean percent time spent freezing during the tone CS (right) but not before the tone (pre-CS freezing, left). (B) No significant correlation was observed between the number of neurons infected with the CREB vector in auditory thalamic regions outside of the MGm/PIN area and the mean percent time spent freezing either before (pre-CS) or during (CS) the tone. (C,D) No significant correlation was observed for the number of neurons infected with the Control vector either in the MGm/PIN region (C) or surrounding thalamic regions (D) and the mean percent time spent freezing during the test.
Conditioned auditory fear memory does not correlate with the number of neurons infected with the CREB vector in surrounding regions of the auditory thalamus
The number of neuronal nuclei in nontarget regions of the auditory thalamus (the MGd/MGv/SG) infected with the CREB vector did not correlate with either pre-CS or CS freezing levels (Fig. 4B). The lines of best fit for these correlations were y = 0.01x + 19.84 and y = 0.02x + 32.98, for pre-CS and CS freezing, respectively, and no significant correlations were found (pre-CS [F(1,20) = 2.38; P > 0.05; R2 = 0.11] and CS [F(1,20) = 0.79; P > 0.05; R2 = 0.04]). Together, these findings indicate that the memory enhancement produced by increasing CREB in the auditory thalamus is mediated by neurons with nuclei in the MGm/PIN region.
Conditioned auditory fear memory does not correlate with the number of neurons infected by the Control vector in the auditory thalamus
As a control, we also correlated the number of neurons with the Control vector in the target region, or surrounding auditory thalamic regions, with freezing levels. Fifteen mice (11 with correct bilateral placements and four with correct unilateral placements) were included in this analysis. As expected, there was no significant relationship between the number of neurons in the MGm/PIN with the Control vector and freezing levels during the tone (best fit line y = −0.04x + 34.51; F(1,13) = 2.63; P > 0.05; R2 = 0.17) or baseline period (y = −0.01x + 10.48; F(1,13) = 0.65; P > 0.05; R2 = 0.05) (Fig. 4C). A similar lack of correlation was observed between the number of neurons in the MGd/MGv/SG with the Control vector and freezing during (y = −0.01x + 33.60; F(1,13) = 0.79; P > 0.05; R2 = 0.06) or before (y = −0.002x + 10.62; F(1,13) = 0.34; P > 0.05; R2 = 0.03) the tone (Fig. 4D). Together, these data indicate that infusion of the Control vector into the MGm/PIN or surrounding thalamic regions does not influence auditory fear memory or freezing behavior in general.
Increasing CREB in the MGm/PIN does not enhance context fear memory
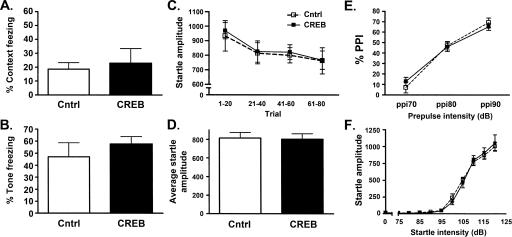
Figure 3C shows that increasing CREB in the MGm/PIN enhances memory for auditory fear conditioning. One interpretation of these data is that increasing CREB in this region facilitates the consolidation of the tone-shock association. Alternatively, increasing CREB in this region may nonspecifically enhance all fear memory. To examine this possibility, we infused an additional group of mice with CREB or Control vector into the MGm/PIN and trained them for context fear conditioning (in the absence of a tone). Mice were placed in the chamber and, 2 min later, a shock (0.4 mA) was delivered. Testing occurred 24 h later when mice were returned to the same context and the percent time mice spent assessed. Figure 5A shows that infusion of the CREB vector into the MGm/PIN does not alter memory for context fear conditioning (F(1,7) = 0.36; P > 0.05, CREB vector [n = 8], Control vector [n = 5]). This lack of enhancement is consistent with previous reports showing that the MGm/PIN region is not critically involved in context fear conditioning (LeDoux et al. 1986; Campeau and Davis 1995a; Parsons et al. 2006). Furthermore, this finding indicates that increasing CREB levels in the MGm/PIN specifically enhances auditory fear memory, rather than conditioned fear memory in general.
Figure 5.
Increasing CREB levels in the MGm/PIN of mice is specific to the formation of auditory fear memory. Increasing CREB levels in the MGm/PIN region does not enhance formation of a context fear memory (A) or expression of an auditory fear memory (B). (C–F) General auditory processing is not changed by increasing CREB levels in the MGm/PIN. Habituation (C) or mean (±SEM) (D) average startle amplitude of the acoustic startle response did not differ in mice that received Cntrl or CREB vector infusions into the MGm/PIN region. (E) Mean (±SEM) percent prepulse inhibition (% PPI) of the acoustic startle response did not differ in mice with increased CREB levels in the MGm/PIN. (F) Increasing CREB levels in the MGm/PIN did not affect acoustic startle threshold.
Increasing CREB in the MGm/PIN does not enhance expression of conditioned auditory fear
A second possibility is that increasing CREB levels in the MGm/PIN enhances expression, rather than formation, of auditory fear memory. To examine this, we trained mice for auditory fear conditioning as before but infused the viral vectors after (rather than before) training. We tested mice 3 d following surgery, at the time of highest transgene expression. Figure 5B shows that infusion of the CREB vector into the MGm/PIN after training does not enhance the expression of an auditory fear memory. This observation is supported by the results of a Vector by Time ANOVA in which there was a significant effect of Time only and, importantly, no significant effect of Vector or Vector-by-Time interaction (Vector-by-Time interaction [F(1,11) = 0.011; P > 0.05] Vector [F(1,11) = 1.79; P > 0.05], Time [F(1,11) = 16.26; P < 0.05], CREB vector [n = 7], Control vector [n = 5]). Bonferroni post-hoc comparisons of the significant Time effect showed that freezing levels in both groups increased during the tone (P < 0.001). Therefore, increasing CREB in the MGm/PIN does not enhance expression of an auditory fear memory.
Increasing CREB in the MGm/PIN does not alter basic auditory processing
Although the results in Figure 3A,B above, suggest that the effects of increasing CREB levels in the MGm/PIN are specific to the formation of an auditory fear memory, it could be that increasing CREB levels in this important region of the auditory pathway enhances the impact of the CS during training. That is, the observed enhancement of auditory fear memory may be due to an increase in salience of the auditory CS during training. To closely examine this possibility, we used the auditory startle response to test the effects of similarly increasing CREB in the MGm/PIN on hearing and sensorimotor processing. First, we found that infusion of the CREB vector did not alter the habituation of the auditory startle response to repeated presentations of a 120-dB auditory stimulus (Fig. 5C). This observation is supported by the results of a Vector-by-Time ANOVA in which there was a significant effect of Time only and, importantly, no significant effect of Vector or Vector-by-Time interaction (Vector-by-Time interaction [F(3,36) = 0.036; P > 0.05], Vector [F(1,12) = 0.049; P > 0.05], Time [F(3,36) = 16.26; P < 0.05], CREB vector [n = 7], Control vector [n = 7]). Bonferroni post-hoc comparisons of the significant Time effect showed that the startle response decreased over trials (P < 0.001). Therefore, increasing CREB in the MGm/PIN did not affect habituation of the acoustic startle response, a nonassociative form of learning (Duerr and Quinn 1982; Hawkins et al. 1998). There was also no effect of increasing CREB on overall startle responding (Fig. 5D). This finding further indicates that this manipulation does not increase auditory startle or anxiety-like behavior.
We used prepulse inhibition to examine the effects of increasing CREB in the MGm/PIN on sensorimotor gating of the auditory startle response. We tested mice with three different prepulse intensities (70, 75, and 80 dB) prior to a 120-dB startle pulse. As can be seen in Figure 5E, the CREB vector did not alter sensorimotor gating. Mice infused with Control or CREB vector showed greater inhibition of the startle response with higher prepulse intensities. The results of an ANOVA supported this interpretation, showing a significant effect of Prepulse Intensity only and, importantly, no significant effect of Vector or Vector × Prepulse Intensity interaction (Vector by Prepulse Intensity interaction [F(2,24) = 1.09; P > 0.05], Vector [F(1,12) = 0.002; P > 0.05], Prepulse Intensity [F(2,24) = 145.79; P < 0.05]).
Finally, to examine whether increasing CREB in the MGm/PIN lowered the auditory threshold for startle responding, we tested the startle amplitude of mice over a range of different intensities of auditory stimuli (ranging from 0 to 120 dB, Fig. 5F). Increasing CREB in the MGm/PIN did not alter the threshold of startle responding, a significant effect of Startle Intensity only and, importantly, no significant effect of Vector or Vector by Startle Intensity interaction (Vector by Startle Intensity interaction [F(10,120) = 0.30; P > 0.05], Vector [F(1,12) = 0.008; P > 0.05], Startle Intensity [F(10,120) = 171.66; P < 0.05]). Bonferroni post-hoc comparisons of the significant Startle Intensity effect showed that the startle response in both groups of mice increased at 100 dB (P < 0.001). Together, these data demonstrate that increasing CREB in the MGm/PIN did not alter numerous aspects of basic central auditory processing. Therefore, the enhancement of auditory fear memory in mice with higher levels of CREB in the MGm/PIN cannot be attributed to changes in simple auditory processing.
Increasing CREB in the MGm/PIN increases the generalization of conditioned auditory fear
Our findings that (1) auditory fear conditioning increases CREB activation in the MGm and PIN and (2) exogenously increasing CREB levels in this region enhances auditory fear conditioning converge to suggest that CREB-mediated plasticity in the MGm/PIN is important for the formation of conditioned auditory fear memory. However, the functional contribution of this plasticity is unknown.
The LA receives both direct and indirect projections from the auditory thalamus. Previous results show that either the thalamo–amygdala pathway (direct projection from the MGm/PIN to the amygdala) or the thalamo–cortico–amygdala pathway (indirect pathway from regions of the auditory thalamus to the cortex to the amygdala) is sufficient to support auditory fear conditioning to a single tone CS (Romanski and LeDoux 1992; Campeau and Davis 1995b). However, each of these pathways may make a unique contribution. Several lines of evidence suggest that the direct thalamo–amygdala pathway processes auditory information in a rapid but crude manner, while the thalamo–cortico–amygdala pathway processes more detailed representations of sound stimuli (LeDoux 1995). First, many cells in the MGm/PIN are broadly tuned (Bordi and LeDoux 1994a, b), in contrast to the sharply tuned cells in the auditory cortex and MGv, a region of the auditory thalamus that directly projects to the auditory cortex, which then projects to the LA (Miller et al. 1972; Rauschecker et al. 1995). Second, post-training lesions of the auditory cortex disrupt performance on a complex sound processing (tone discrimination) task (Thompson 1960; Jarrell et al. 1987). It is somewhat surprising, therefore, that pretraining lesions of the auditory cortex failed to influence tone fear generalization in both a computational model and behaving rats (Armony et al. 1997). These apparently discrepant results could be due to differences between mechanisms that mediate tone discrimination and generalization, the timing of lesion (pre-versus post-training), and potential functional compensation by other intact brain areas following the lesion. As an alternative strategy, we examined the effects by using a gain-of-function approach.
Because many cells in the MGm/PIN are broadly tuned, we examined whether increasing CREB in this region increased tone fear generalization. To this end, a random subset of mice injected with the CREB vector into the MGm/PIN (n = 7, from Fig. 3C) was given two additional tone generalization tests during which freezing levels to different frequency tones (700 and 10,000 Hz, rather than CS training tone [2800 Hz]) were measured. The order of tone presentation (700, 10,000 Hz) in these generalization tests was counterbalanced across mice. Because mice that were infused with Control vector did not show high levels of freezing to the training CS tone (see Fig. 3C), we did not test them further for tone fear generalization. Instead, we compared tone generalization in the CREB vector group to an additional unoperated control group (n = 19) trained using a protocol that produced similar levels of CS freezing (2800-Hz tone paired with a 0.5-mA shock; see Fig. 2A).
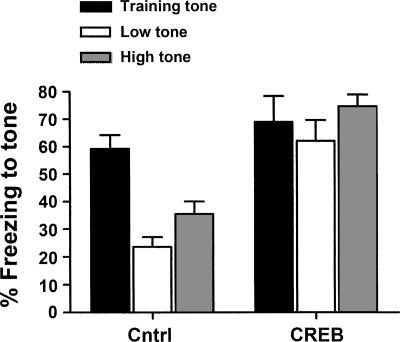
Figure 6 shows the percent time mice spent freezing to the training tone (2800 Hz), as well the low-(700 Hz) and high-(10,000 Hz) frequency tones. As can be seen from this graph, the control mice froze much less to the high- and low-frequency tones than to the training tone, showing little tone fear generalization. In contrast, mice infused with the CREB vector froze at equally high levels to the training and the other tones, showing enhanced tone fear generalization. The results of an ANOVA (Group × Tone Frequency) supported this finding, revealing a significant interaction of Group-by-Tone Frequency (F(2,48) = 4.84; P < 0.05) as well as significant main effects of Group (F(1,24) = 25.10; P < 0.001) and Tone Frequency (F(2,48) = 7.86; P < 0.05). Bonferroni post-hoc analysis of the significant interaction indicated that freezing to the training tone was similar in both groups. Furthermore, whereas the control mice froze significantly less to the low- and high-frequency tones than to the training tone, mice infused with the CREB vector froze at similarly high levels to all tone frequencies. Therefore, increasing CREB in the MGm/PIN broadens the generalization gradient for conditioned auditory fear.
Figure 6.
Increasing CREB levels in the MGm/PIN of mice enhances generalization of conditioned tone fear. Mean (±SEM) percent time mice spent freezing in the test sessions when presented with tones of different frequencies. Control mice showed little tone fear generalization as they froze much more to the training tone frequency (2800 Hz) than to tones of both higher (10,000 Hz) and lower (700 Hz) frequencies. In contrast, mice infused with the CREB vector showed equally high levels to the training and other tones.
Discussion
The present experiments examined the role of the CREB-mediated plasticity in MGm/PIN in conditioned auditory fear memory. We targeted this region based on three key findings. First, antidromic stimulation of the LA activates cells in the MGm/PIN, indicating that this region sends direct projections to the LA (Bordi and LeDoux 1994b). Second, cells in the MGm/PIN receive inputs from both auditory and somatosensory pathways (Bordi and LeDoux 1994b), providing an anatomical substrate for possible CS/US convergence. Surrounding regions of the auditory thalamus do not share these important characteristics. For instance, cells in the MGv fail to respond to both auditory and somatosensory stimulation and few or no antidromically activated cells are observed in the MGv, SG, or MGd following LA stimulation (Bordi and LeDoux 1994b). Finally, labeling studies reveal that the PIN is the primary source of the direct thalamo–LA pathway (Doron and Ledoux 2000).
It is well established that enduring forms of memory depend on mRNA and protein synthesis. Accordingly, previous studies examined the role of plasticity in the MGm/PIN on conditioned auditory fear memory by locally infusing drugs that interfere with these processes. Intra-thalamic infusions of RNA synthesis inhibitors either before or after training specifically block long-term memory for auditory fear conditioning (Apergis-Schoute et al. 2005; Parsons et al. 2006). However, the effect of anisomycin (a drug used to interfere with translation) seems to vary with the time of infusion. Long-term memory is impaired when anisomycin is infused before training (Parsons et al. 2006), but not after training (Maren et al. 2003; Apergis-Schoute et al. 2005). These contrasting results may be due to several factors, such as the nonspecific effects of anisomycin (Zechner et al. 1997) or a disparity between the time course of protein synthesis disruption produced by this manipulation and the time window of protein synthesis necessary to support long-term memory in this region. Furthermore, because anisomycin may disrupt protein synthesis in neurons both within and surrounding the MGm/PIN, it is difficult to localize the precise anatomical regions mediating any potential effect.
We used a gain-of-function approach to examine the role and behavioral significance of plasticity in the MGm/PIN in conditioned auditory fear memory. We found that auditory fear conditioning increased the levels of activated CREB in both the MGm and PIN. Control training manipulations did not produce similar increases in pCREB, thus highlighting the specificity of the observed increase in CREB activation. These findings indicate that training that induces long-term memory for auditory fear conditioning normally activates CREB in the MGm and PIN. Next, we therefore examined the effects of exogenously increasing CREB in this region.
We showed that increasing CREB levels in the MGm/PIN enhanced memory for auditory fear conditioning. We trained mice using a low intensity of shock that normally produced weak memory for auditory conditioned fear. However, mice infused with the CREB vector into the MGm/PIN showed robust auditory fear memory. Tagging the CREB transgene with GFP allowed us to localize the specific brain regions mediating this memory enhancement. We found that the number of neuronal nuclei in the MGm/PIN (but not surrounding regions of the auditory thalamus) with the CREB vector was positively correlated with the level of auditory fear memory. Therefore, the memory enhancement produced by increasing CREB levels can be specifically attributed to neurons with nuclei located in the MGm/PIN.
In a series of control studies we examined the specificity of this memory enhancement. We showed that increasing CREB in the MGm/PIN did not alter (1) expression of an auditory fear memory, (2) hearing or auditory processing (as measured by habituation, prepulse inhibition, or threshold of the acoustic startle response), or (3) formation of a context fear memory. Therefore, the auditory fear memory enhancement produced by increasing CREB in the MGm/PIN cannot be attributed to an increase in overall fear memory, a nonspecific increase in anxiety, or a general change in auditory processing.
The present results are consistent with previous findings showing that intra-thalamic infusion of U0126, a MEK inhibitor, prior to training impaired long-term memory for auditory fear conditioning (Apergis-Schoute et al. 2005). Because MEK phosphorylates CREB via MAPK and RSK (Roberson et al. 1999), it is tempting to speculate that the memory-impairing effects of U0126 were mediated by disrupting CREB function.
The present findings are also in agreement with the notion that the MGm/PIN region stores critical aspects of a tone-reward memory. Following tone-reward pairing in mice, presentation of the tone alone produced a region-specific increase in high-frequency firing in MGm/PIN neurons (Komura et al. 2001). Extinction training gradually decreased this neuronal firing. However, a single retraining trial was sufficient to rapidly restore the previously observed high-frequency firing in the MGm/PIN. The rapidity of this response suggests that the MGm/PIN stores some aspect of the tone-reward memory. Together, these results show a conserved role for the MGm/PIN in learning during which a tone acquires emotional significance (either aversive and appetitive).
The present findings that the MGm/PIN is important for auditory fear conditioning memory do not diminish the role of plasticity in the LA. Indeed, we found that increasing CREB function in the LA produced a similar robust memory enhancement. Although the role of LA plasticity in the consolidation of auditory conditioned fear memory has been clearly established (Davis 1992; Fanselow and LeDoux 1999; LeDoux 2000; Maren and Quirk 2004; Dityatev and Bolshakov 2005; but see Cahill et al. 1999), accumulating data indicate that plasticity in multiple regions may be necessary for long-term auditory fear memory (Pare et al. 2004; Wilensky et al. 2006).
Finally, we investigated the functional role of MGm/PIN plasticity in conditioned auditory fear. We found that increasing CREB in the MGm/PIN increased tone fear generalization. Control mice showed a sharp tone fear generalization gradient (they froze robustly to the CS tone frequency but much less to other tones). In contrast, mice infused with the CREB vector froze at equally high levels to the CS tone and to the tones of both higher and lower frequencies. We controlled for overall levels of fear by training control mice with a higher intensity of shock (0.5-mA shock) than mice infused with the CREB vector (trained with a 0.4-mA shock). Indeed, the levels of freezing to the CS tone frequency did not differ between these groups, indicating that the overgeneralization of tone fear in mice infused with the CREB vector cannot be attributed to a general increase in auditory fear. These results suggest that, although the strength of the memory between control mice and CREB-injected mice with a lower intensity of shock is similar, the quality of the memory is different. The memory produced by increasing CREB in the MGm/PIN is less precise. Interestingly, mice infused with the CREB vector did not show enhanced freezing during the pre-CS period (Fig. 3C) or to a context previously paired with shock (Fig. 5A). Together, these findings indicate that the overgeneralization produced by increasing CREB in the MGm/PIN is specific to intra-dimensional (auditory) stimuli.
The finding that increasing CREB in the MGm/PIN increased tone fear generalization is consistent with the broad receptive field plasticity that develops in the MGm/PIN region during auditory fear conditioning. Although cells in the thalamo–amygdala pathway (specifically the MGm) and thalamo–cortico–amygdala (specifically the MGv and auditory cortex) pathways often “retune” their frequency receptive fields to increase responding to the CS frequency (Weinberger 2007) during training, the precision of this retuning is region-specific. Cells in the auditory cortex (Bakin and Weinberger 1990) or MGv (Edeline and Weinberger 1991) typically develop a single sharp-peaked best firing frequency curve centered near the CS frequency. In contrast, the more broadly tuned cells in the MGm develop a generalized multi-peaked curve that responds to many tone frequencies (Edeline and Weinberger 1992, 1993). Our current findings that increasing CREB in the MGm/PIN broadens tone fear generalization are in agreement with the broad retuning curves normally observed in this region.
In normal rodents, an increase in auditory fear generalization is observed following overtraining (deToledo 1971; Laxmi et al. 2003). Together with the present data that increasing CREB levels in the MGm/PIN similarly broadens tone fear generalization, these findings suggest that overtraining in normal rodents increases CREB function in the MGm/PIN. Furthermore, these results support the notion that, rather than being a simple lack of differentiation, fear generalization is an active process of judging different sensory stimuli as being sufficiently similar to predict danger (Shepard 1987).
The neural substrates underlying the acquisition and consolidation of memory for auditory fear conditioning have been well studied. In contrast, relatively little is known about the neural mechanisms that determine the degree to which conditioned auditory fear generalizes to similar tones. The present findings suggest that CREB-mediated plasticity in the MGm/PIN is important for long-term memory for auditory fear conditioning and regulating the extent of auditory fear generalization.
Materials and Methods
Mice
Adult F1 hybrid (C57Bl/6NTac × 129S6/SvEvTac) mice were group-housed (three to five mice per cage) on a 12-h light/dark cycle. Food and water were available ad libitum throughout the experiment. All procedures were approved by the Hospital for Sick Children Animal Care and Use Committee.
Immunocytochemistry
Male mice aged 3–5 mo were handled for six consecutive days and randomly assigned to the following treatment groups: (1) Tone+Shock (Tone fear conditioning [Tone FC, n = 6]), (2) Tone alone (n = 4), (3) Chamber+Shock (Context FC, n = 4), (4) Immediate Shock (n = 4), (5) Chamber alone (n = 4), and (6) Homecage (n = 4). The shock intensity for all mice receiving shock in these immunocytochemical experiments was 0.5 mA. Mice in the Tone+Shock group were placed in a conditioning chamber (Context A) and, 2 min later, presented a tone (2800 Hz, 85 dB, 30 sec) that coterminated with a shock (2 sec, 0.5 mA). Mice in the Tone alone group were treated identically, except that no shock was delivered. Two minutes following placement in the chamber, mice in the Chamber+Shock (context fear conditioning) group received the shock (without tone). The Immediate shock group received the shock 5 sec after being placed in the chamber and was not exposed to the tone. The Chamber alone group did not receive the tone or the shock following placement in the chamber. The Homecage mice were taken directly from their homecages and not exposed to the conditioning chamber, tone, or shock.
Thirty minutes following training, mice were perfused transcardially with 4% paraformaldehyde. Brains were sliced coronally (50 μm) and prepared for immunocytochemistry using anti-pCREB primary rabbit polyclonal antibody (1:1000 dilution, Upstate Cell Signaling Solutions). We chose this 30-min time-point based on previous studies (Trifilieff et al. 2006). A biotinylated goat anti-rabbit antibody (1:1000 dilution, Vector Laboratories) was used as a secondary antibody. Staining was visualized using the avidin-biotin peroxidase method (Vectastain Elite ABC kit, Vector Laboratories) coupled to diaminobenzidine (DAB, Sigma) as a chromogen. No staining was detected in the absence of the primary or secondary antibodies. Quantitative analysis of pCREB-positive nuclei was performed using the NIH Image processing system by two experimenters unaware of the treatment condition. The total number of immunoreactive cells in the MGm and PIN (as defined by Paxinos and Franklin 2003) were counted bilaterally from at least six sections from comparable anteroposterior levels from each mouse. The number of pCREB positive nuclei (per 0.1 mm2 of MGm and PIN tissue, respectively) was calculated per mouse and averaged for each group.
HSV vectors
Two vectors were used, HSV-GFP-CREB (CREB vector) and HSV-GFP-LacZ (Control vector). Genes of interest (CREB, LacZ) were cloned into the HSV amplicon (HSV-PrpUC) and packaged using a replication-defective helper virus (with a 5dl1.2 deletion). To visualize transgene expression, we fused eGFP to the 5′ end of the CREB and LacZ cDNA. Transgene expression was regulated by the constitutive promoter for the HSV immediate-early gene IE 4/5. Virus was purified on a sucrose gradient, pelleted, and resuspended in 10% sucrose. The average titer of the recombinant virus stocks was typically 4.0 × 107 infectious units/mL. Previous studies established tagging CREB with GFP does not interfere with this functional activity (Chao et al. 2002).
Validation of vectors
To ensure that infusion of the CREB vector indeed enhanced CREB levels in the MGm/PIN, we performed immunocytochemistry using an anti-CREB primary mouse monoclonal antibody (1:1000, Upstate Cell Signaling Solutions). A biotinylated donkey anti-mouse antibody (1:1000, Jackson ImmunoResearch) was used as a secondary antibody. Immunoreactivity was visualized using a DAB reaction as above.
Surgery
Mice were pretreated with atropine sulfate (0.1 mg/kg, i.p.), anesthetized with chloral hydrate (400 mg/kg, i.p.), and placed in a stereotaxic frame. The skin was retracted and holes drilled in the skull bilaterally above the LA (AP = −1.4, ML = ±3.5, V = −5.0 mm from bregma) or MGm/PIN (AP = −3.0, ML = ±2.1, V = −3.1 mm from bregma) according to Paxinos and Franklin (2003). Bilateral microinjections (1.5 μL) of the CREB or Control vector were delivered over 20 min through glass micropipettes. Micropipettes were left in place an additional 10 min to ensure diffusion of the vector. Because transgene expression using this viral system peaks 3 d following surgery (Barrot et al. 2002), we trained mice 3 d following surgery, except in the expression experiment. To examine the effects of increasing CREB in the MGm/PIN on expression of an auditory fear memory, mice were trained and surgery was performed 2 d later. Mice were tested 3 d following surgery.
Auditory (tone) fear conditioning
Training consisted of placing mice in a conditioning chamber (Context A) and, 2 min later, presenting a tone (2800 Hz, 85 dB, 30 sec) that coterminated with a shock (2 sec, 0.3, 0.4, or 0.5 mA, depending on experiment). Mice remained in the chamber for an additional 30 sec. Testing for auditory fear conditioning occurred 24 h later. Mice were placed in a novel chamber (Context B) and 2 min later the tone CS was presented (for 1 min). Our index of memory, freezing (the cessation of all movement except for respiration), was assessed via automated procedures (Actimetrics).
Tone fear generalization test
A subset of CREB-infused and unoperated control mice was further tested for generalization of tone fear conditioning using tones of frequencies different than the training frequency. The control mice used in this generalization experiment were trained with a single tone-shock pairing as above, using a 0.5-mA shock. Twenty-four hours following the initial CS tone test (2800 Hz), mice were placed back in Context B and presented with a similar 85-dB tone that was either higher (10,000 Hz) or lower (700 Hz) in frequency than the training tone. Mice were then transported back to their homecages. Six hours later, mice were once again placed in Chamber B and the tone of the other frequency was played. The order of tone presentation (high versus low frequency) in the tone generalization tests was counterbalanced across mice.
Context fear conditioning
CREB- or Control vector-infused mice were placed in Context A and 2 min later presented with a shock (2 sec, 0.4 mA). Mice remained in the context for an additional 30 sec. Twenty four hours later, mice were replaced in the context and the amount of time spent freezing during the 3-min test was assessed.
Auditory startle response
Startle testing was conducted using a SR-LAB startle testing system (San Diego Instruments). Mice were placed in a Plexiglas testing cylinder (3.2 cm internal diameter). Acoustic startle stimuli and prepulse stimuli were delivered via a high-frequency speaker, placed 15 cm from the testing cylinder. Background white noise was generated by a standard speaker. The testing cylinder was mounted on a sensor platform. A piezoelectric accelerometer, attached to the base of the sensor platform, detected and transduced cage movements that were then digitized by and stored in a computer. The startle amplitude was taken to be the maximal response that occurred in the 100 msec after presentation of the startle stimulus. The sound levels for background noise and startle/prepulse stimuli were calibrated with a digital sound level meter. The speakers, testing cylinder, and sensor platform were housed within a sound-attenuated chamber.
Habituation
Mice were placed in the testing cylinder and, 5 min later, presented with 80 startle pulses of 120 dB each (15 sec interstimulus interval [ISI]).
Prepulse inhibition
The next day, mice were tested for prepulse inhibition of the startle response. Following a 5-min acclimation period where no stimuli were delivered, mice were presented with 20 habituation trials (120 dB, ISI 15 sec). In the prepulse inhibition phase, mice were presented with a total of 90 trials. Three prepulse intensities were tested: 70, 75, and 80 dB. Prepulses were 20 msec in duration with a rise/fall time of <1 msec. For each prepulse intensity, there were three types of trial: prepulse alone, prepulse/startle stimulus, and startle stimulus alone. In the prepulse/startle stimulus trial, the onset of the prepulse preceded the onset of the startle stimulus by 100 msec. All startle stimuli were presented in a pseudorandom sequence with the constraint that each stimulus intensity occur only once in each consecutive four-trial block. The % PPI was calculated per mouse for each of the three prepulse conditions.
Startle threshold
The following day, mice were given a startle threshold test session. Following an acclimation period of 5 min, mice were presented with a total of 99 trials (15-sec ISI). There were 11 trial types: no stimulus (NS), and 10 types of startle trials in which the intensity of the startle stimulus varied from 75 to 120 dB (with 5 dB increments). The startle stimuli were 40 msec noise bursts with a rise/fall time of <1 msec. The 11 trial types (NS, startle stimuli) were presented in a pseudorandom order such that each trial type was presented once within a block of 11 trials. Startle threshold was defined as the minimal intensity at which responding was significantly greater than in the NS trials.
Histology
Placement and extent of the viral infection were determined by GFP-immunofluorescence. Only those mice that showed robust bilateral expression of GFP in the target region (LA or MGm/PIN, depending on the experiment) were included in subsequent statistical analysis.
Acknowledgments
We thank Paul W. Frankland for helpful comments on a previous draft of this manuscript; Ivan Ho, Ricky Sheth, and Nicodemus Oey for technical assistance; and Asim Rashid for expert advice on this project. This project was supported by CIHR (MOP 74650) and NSERC grants to S.A.J. J.H.H., A.P.Y., and C.J.C. are supported by Restracomp Fellowships from the Hospital for Sick Children.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.993608.
References
- Anagnostaras S.G., Josselyn S.A., Frankland P.W., Silva A.J. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn. Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apergis-Schoute A.M., Debiec J., Doyere V., LeDoux J.E., Schafe G.E. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: A role for presynaptic plasticity in the fear system. J. Neurosci. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony J.L., Servan-Schreiber D., Romanski L.M., Cohen J.D., LeDoux J.E. Stimulus generalization of fear responses: Effects of auditory cortex lesions in a computational model and in rats. Cereb. Cortex. 1997;7:157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Bailey D.J., Kim J.J., Sun W., Thompson R.F., Helmstetter F.J. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav. Neurosci. 1999;113:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Bakin J.S., Weinberger N.M. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Balschun D., Wolfer D.P., Gass P., Mantamadiotis T., Welzl H., Schutz G., Frey J.U., Lipp H.P. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M., Olivier J.D., Perrotti L.I., DiLeone R.J., Berton O., Eisch A.J., Impey S., Storm D.R., Neve R.L., Yin J.C., et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F., LeDoux J.E. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp. Brain Res. 1994a;98:261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Bordi F., LeDoux J.E. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp. Brain Res. 1994b;98:275–286. doi: 10.1007/BF00228415. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Cahill L., Weinberger N.M., Roozendaal B., McGaugh J.L. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Campeau S., Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J. Neurosci. 1995a;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S., Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J. Neurosci. 1995b;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon W.A., Neve R.L. Viral-mediated gene transfer to study the behavioral correlates of CREB function in the nucleus accumbens of rats. Methods Mol. Med. 2003;79:331–350. doi: 10.1385/1-59259-358-5:331. [DOI] [PubMed] [Google Scholar]
- Chao J.R., Ni Y.G., Bolanos C.A., Rahman Z., DiLeone R.J., Nestler E.J. Characterization of the mouse adenylyl cyclase type VIII gene promoter: Regulation by cAMP and CREB. Eur. J. Neurosci. 2002;16:1284–1294. doi: 10.1046/j.1460-9568.2002.02186.x. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc. Natl. Acad. Sci. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K., Hochner B., Kandel E.R. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Desmedt A., Hazvi S., Dudai Y. Differential pattern of cAMP response element-binding protein activation in the rat brain after conditioned aversion as a function of the associative process engaged: Taste versus context association. J. Neurosci. 2003;23:6102–6110. doi: 10.1523/JNEUROSCI.23-14-06102.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deToledo L. Changes in heart rate during conditioned suppression in rats as a function of US intensity and type of CS. J. Comp. Physiol. Psychol. 1971;77:528–538. doi: 10.1037/h0031872. [DOI] [PubMed] [Google Scholar]
- Dityatev A.E., Bolshakov V.Y. Amygdala, long-term potentiation, and fear conditioning. Neuroscientist. 2005;11:75–88. doi: 10.1177/1073858404270857. [DOI] [PubMed] [Google Scholar]
- Doron N.N., Ledoux J.E. Cells in the posterior thalamus project to both amygdala and temporal cortex: A quantitative retrograde double-labeling study in the rat. J. Comp. Neurol. 2000;425:257–274. [PubMed] [Google Scholar]
- Duerr J.S., Quinn W.G. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proc. Natl. Acad. Sci. 1982;79:3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline J.M., Weinberger N.M. Thalamic short-term plasticity in the auditory system: Associative returning of receptive fields in the ventral medial geniculate body. Behav. Neurosci. 1991;105:618–639. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- Edeline J.M., Weinberger N.M. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: Receptive field plasticity in the medial division of the medial geniculate body. Behav. Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Edeline J.M., Weinberger N.M. Receptive field plasticity in the auditory cortex during frequency discrimination training: Selective retuning independent of task difficulty. Behav. Neurosci. 1993;107:82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Gale G.D. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., LeDoux J.E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fink D.J., DeLuca N.A., Goins W.F., Glorioso J.C. Gene transfer to neurons using herpes simplex virus-based vectors. Annu. Rev. Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- Frank D.A., Greenberg M.E. CREB: A mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Gerren R.A., Weinberger N.M. Long term potentiation in the magnocellular medial geniculate nucleus of the anesthetized cat. Brain Res. 1983;265:138–142. doi: 10.1016/0006-8993(83)91344-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez G.A., Montminy M.R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Graves L., Dalvi A., Lucki I., Blendy J.A., Abel T. Behavioral analysis of CREB alphadelta mutation on a B6/129 F1 hybrid background. Hippocampus. 2002;12:18–26. doi: 10.1002/hipo.10003. [DOI] [PubMed] [Google Scholar]
- Guzowski J.F., McGaugh J.L. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.H., Kushner S.A., Yiu A.P., Cole C.J., Matynia A., Brown R.A., Neve R.L., Guzowski J.F., Silva A.J., Josselyn S.A. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Hawkins R.D., Cohen T.E., Greene W., Kandel E.R. Relationships between dishabituation, sensitization, and inhibition of the gill- and siphon-withdrawal reflex in Aplysia californica: Effects of response measure, test time, and training stimulus. Behav. Neurosci. 1998;112:24–38. doi: 10.1037//0735-7044.112.1.24. [DOI] [PubMed] [Google Scholar]
- Impey S., Smith D.M., Obrietan K., Donahue R., Wade C., Storm D.R. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat. Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Jarrell T.W., Gentile C.G., Romanski L.M., McCabe P.M., Schneiderman N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardiac conditioning to acoustic conditioned stimuli in rabbits. Brain Res. 1987;412:285–294. doi: 10.1016/0006-8993(87)91135-8. [DOI] [PubMed] [Google Scholar]
- Jasnow A.M., Shi C., Israel J.E., Davis M., Huhman K.L. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav. Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Josselyn S.A., Shi C., Carlezon W.A., Neve R.L., Nestler E.J., Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn S.A., Kida S., Silva A.J. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol. Learn. Mem. 2004;82:159–163. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Kapp B.S., Frysinger R.C., Gallagher M., Haselton J.R. Amygdala central nucleus lesions: Effect on heart rate conditioning in the rabbit. Physiol. Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kassubek J., Juengling F.D., Ecker D., Landwehrmeyer G.B. Thalamic atrophy in Huntington's disease co-varies with cognitive performance: A morphometric MRI analysis. Cereb. Cortex. 2005;15:846–853. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- Kida S., Josselyn S.A., de Ortiz S.P., Kogan J.H., Chevere I., Masushige S., Silva A.J. CREB required for the stability of new and reactivated fear memories. Nat. Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Komura Y., Tamura R., Uwano T., Nishijo H., Kaga K., Ono T. Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature. 2001;412:546–549. doi: 10.1038/35087595. [DOI] [PubMed] [Google Scholar]
- Lamprecht R., Hazvi S., Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J. Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmi T.R., Stork O., Pape H.C. Generalisation of conditioned fear and its behavioural expression in mice. Behav. Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion: Clues from the brain. Annu. Rev. Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Iwata J., Pearl D., Reis D.J. Disruption of auditory but not visual learning by destruction of intrinsic neurons in the rat medial geniculate body. Brain Res. 1986;371:395–399. doi: 10.1016/0006-8993(86)90383-5. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Farb C., Ruggiero D.A. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J. Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Quirk G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S., Yap S.A., Goosens K.A. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J. Neurosci. 2001;21:RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. http://www.jneurosci.org/cgi/content/abstract/21/6/RC135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Ferrario C.R., Corcoran K.A., Desmond T.J., Frey K.A. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur. J. Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- McAlonan K., Cavanaugh J., Wurtz R.H. Attentional modulation of thalamic reticular neurons. J. Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron M.D., Green E.J., Winters R.W., Nolen T.G., Schneiderman N., McCabe P.M. Changes of synaptic efficacy in the medial geniculate nucleus as a result of auditory classical conditioning. J. Neurosci. 1996;16:1273–1283. doi: 10.1523/JNEUROSCI.16-03-01273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.M., Sutton D., Pfingst B., Ryan A., Beaton R., Gourevitch G. Single cell activity in the auditory cortex of Rhesus monkeys: Behavioral dependency. Science. 1972;177:449–451. doi: 10.1126/science.177.4047.449. [DOI] [PubMed] [Google Scholar]
- Minamimoto T., Hori Y., Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science. 2005;308:1798–1801. doi: 10.1126/science.1109154. [DOI] [PubMed] [Google Scholar]
- Neve R.L., Neve K.A., Nestler E.J., Carlezon W.A. Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39:381–391. doi: 10.2144/05393PS01. [DOI] [PubMed] [Google Scholar]
- Olson V.G., Zabetian C.P., Bolanos C.A., Edwards S., Barrot M., Eisch A.J., Hughes T., Self D.W., Neve R.L., Nestler E.J. Regulation of drug reward by cAMP response element-binding protein: Evidence for two functionally distinct subregions of the ventral tegmental area. J. Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D., Quirk G.J., Ledoux J.E. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parsons R.G., Riedner B.A., Gafford G.M., Helmstetter F.J. The formation of auditory fear memory requires the synthesis of protein and mRNA in the auditory thalamus. Neuroscience. 2006;141:1163–1170. doi: 10.1016/j.neuroscience.2006.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2003. [Google Scholar]
- Pittenger C., Huang Y.Y., Paletzki R.F., Bourtchouladze R., Scanlin H., Vronskaya S., Kandel E.R. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Farb C.R., He Y., Janssen W.G., Rodrigues S.M., Johnson L.R., LeDoux J.E., Morrison J.H. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J.P., Tian B., Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Roberson E.D., English J.D., Adams J.P., Selcher J.C., Kondratick C., Sweatt J.D. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J. Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski L.M., LeDoux J.E. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J. Neurosci. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G.E., LeDoux J.E. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Greenberg M.E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Shepard R.N. Toward a universal law of generalization for psychological science. Science. 1987;237:1317–1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Simone D.A., Zhang X., Li J., Zhang J.M., Honda C.N., LaMotte R.H., Giesler G.J. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J. Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Stanciu M., Radulovic J., Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: Relationship to Fos production. Brain Res. Mol. Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Thompson R.F. Function of auditory cortex of cat in frequency discrimination. J. Neurophysiol. 1960;23:321–334. doi: 10.1152/jn.1960.23.3.321. [DOI] [PubMed] [Google Scholar]
- Trifilieff P., Herry C., Vanhoutte P., Caboche J., Desmedt A., Riedel G., Mons N., Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn. Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagatsuma A., Azami S., Sakura M., Hatakeyama D., Aonuma H., Ito E. De Novo synthesis of CREB in a presynaptic neuron is required for synaptic enhancement involved in memory consolidation. J. Neurosci. Res. 2006;84:954–960. doi: 10.1002/jnr.21012. [DOI] [PubMed] [Google Scholar]
- Wallace T.L., Stellitano K.E., Neve R.L., Duman R.S. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol. Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Weinberger N.M. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear. Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky A.E., Schafe G.E., Kristensen M.P., LeDoux J.E. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.C., Del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Zechner D., Thuerauf D.J., Hanford D.S., McDonough P.M., Glembotski C.C. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J. Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]