Abstract
In addition to its role as a neurotransmitter, dopamine can stimulate neurite outgrowth and morphological effects upon primary neurons. To investigate the signal transduction mechanisms used by dopamine in developing striatal neurons, we focused upon the effects of activating the dopamine D1 receptor. Using the D1 receptor agonist SKF38393, we found that Trk neurotrophin receptors were activated in embryonic day 18 striatal neurons. K-252a, a Trk tyrosine kinase inhibitor, and a dopamine D1 receptor antagonist could block the effects of SKF38393. The increase in TrkB phosphorylation was not the result of increased neurotrophin production. Induction of TrkB activity by SKF38393 was accompanied by the phosphorylation of several Trk signaling proteins, including phospholipase Cγ, Akt, and MAPK. Biotinylation experiments followed by immunostaining by phospho-TrkB-specific antibodies indicated that the mechanism involved increased TrkB surface expression by dopamine D1 receptor activation. This increase in cell surface TrkB expression was dependent upon an increase in intracellular Ca2+. These results indicate that stimulation of dopamine D1 receptors can be coupled to the neurotrophin receptor signaling to mediate the effects of dopamine upon striatal neurons.
Dopamine, the major neurotransmitter released from dopaminergic neurons, modulates neuronal activity (1–3) and influences key physiological functions related to locomotor activity, reward, and cognition (4, 5). Dopamine also appears to exert several developmental roles. In the lateral ganglionic eminence, dopamine receptors modulate the cell cycle of progenitor cells (6). Dopamine regulates neuronal differentiation and maturation, such as neurite extension and development of growth cones (7–9). However, the molecular mechanisms for these developmental activities have not yet been defined.
Dopamine receptors are classified as D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors (10). Activation of D1-like receptors enhances L-type calcium ion (Ca2+) channel activity and increases intracellular Ca2+ concentration (11–13). Dopamine receptors are G protein-coupled receptors (GPCRs)3 that regulate the signaling results in cyclic 3′-5′ AMP (cAMP) accumulation because of coupling with the heterotrimeric G protein subunits (14, 15). A number of GPCRs can transactivate receptor tyrosine kinases. This suggests that dopamine receptors may regulate trophic effects more broadly by using transactivation of other receptors.
Neurotrophins, such brain-derived neurotrophic factor (BDNF) and neurotrophin-3, are widely expressed in cortex, cerebellum, and hippocampus and have well established effects upon the differentiation and development of many neuronal populations in the central nervous system (16). In addition to neurotrophin binding to Trk receptors, it has been appreciated that Trk receptors can be transactivated by ligands that use GPCRs (17, 18). Transactivation of Trk receptors has been shown to account for neuroprotection and neuronal migration (19, 20).
In this article, we report that D1 receptor stimulation can lead to transactivation of TrkB receptor activity in rat striatal neurons in vitro and in vivo. The activation of D1 receptors results in the regulation of calcium influx and TrkB surface expression in primary striatal cultures. Our results suggest that D1 receptor mediates its signaling by transactivation of TrkB and imply that the development of striatal neurons can depend upon transactivation mechanisms.
EXPERIMENTAL PROCEDURES
Primary Culture—Striatal tissues were dissected from embryos of Sprague-Dawley rats (embryonic days 18–19, Charles River Laboratories, Inc., Wilmington, MA) and dissociated with 0.01% trypsin solution. The neurons were plated at a density of ∼1.0 × 106 cells/ml in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and grown for 5–6 days in serum-free conditions, as described previously (21). Glial cells were plated in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, and grown in 10% serum condition. On days in vitro (DIV) 5 or 6, the cultures were treated with dopamine agonists: SKF38393 (1 μm), quinpirole (1 μm; Sigma-Aldrich Corp.), ionomycin (10 μm; Calbiochem, San Diego, CA), or BDNF (5 ng/ml, Peprotech Inc., Rocky Hill, NJ). Prior to the dopamine agonist treatment, the cultures were preincubated with the dopamine D1 receptor antagonist SCH23390 (1 μm; Sigma) or K-252a (100 nm; Calbiochem), TrkB-Fc (10 μg; Chemicon, Temecula, CA), BAPTA (10 μm), or BAPTA-AM (10 μm, Calbiochem). All of the animal experiments were performed in accordance with the Division of Laboratory Animal Resources Guidelines of New York University School of Medicine.
Surface Biotinylation Assay—The cultures were treated with 1 μm SKF38393 for 3 h and then incubated with 1 mg/ml sulfo-NHS-LC-biotin (Pierce) in phosphate-buffered saline (PBS) containing 1 mm CaCl2 and 1 mm MgCl2 for 30 min on ice (18). The cell lysates were incubated with immobilized streptoavidin-beaded agarose (ImmunoPure; Pierce) overnight at 4 °C. Biotinylated proteins were eluted with 2% SDS buffer at 100 °C and processed for Western blotting analysis.
Immunoprecipitation and Western Blot Analysis—Levels of phospho-TrkB, TrkB, phosphotyrosine were determined by immunoblot analysis using methods similar to those described previously (22). Protein samples for immunoprecipitation were prepared from culture cells or striatal tissues lysate by radioimmune precipitation assay buffer (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% deoxycholate, 0.1% SDS). For immunoprecipitation, the protein lysates were incubated with 2 μg of Trk antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or TrkB antibody (Cell Signaling Technology, Danvers, MA) overnight at 4 °C. The resulting immunocomplexes were precipitated with protein A-Sepharose beads (Amersham Biosciences). After denaturing with 2% SDS, the samples were separated by SDS-PAGE and transferred electrophoretically to polyvinylidine fluoride membranes (Immobilon-P®, Millipore, Bedford, MA). The membranes were probed with Trk antibody (1:1000; Santa Cruz), phosphotyrosine antibody (1:1000; Upstate Biotechnology Inc., Lake Placid, NY), TrkB antibody (1:1000; Cell Signaling), phospho-MAPK antibody (1:1000), MAPK antibody (1:1000), phospho-ARMS antibody (1:500), ARMS antibody (1:2000 (23), phospho-Akt antibody (1:1000), Akt antibody (1:1000; Cell Signaling), neuron-specific enolase (NSE) antibody (1:1000; Chemicon), GAD67 (glutamate decarboxylases of 67 kDa) antibody (1:500; Santa Cruz), glial fibrillary acidic protein antibody (1:1000; Chemicon), or actin antibody (1:5000; Chemicon). After extensive washing, the immunoreactivity on the membranes was detected with anti-rabbit/mouse immunoglobulins conjugated to horseradish peroxidase, followed by a chemiluminescence reaction (ECL kit; Amersham Biosciences). The band intensities were quantified by densitometry.
The specificity of phospho-TrkB antibody was confirmed by peptide competition assay (23). For the competition, we combined phospho-TrkB antibody with a 5-fold excess of TrkB peptide (LQNLAKSPVTLDIC) or phospho-TrkB peptide (LQNLAKSPVT(PO3H2)LDIC) in 500 μl of PBS and incubated overnight at 4 °C, and then we used them for Western blotting.
Immunocytochemistry—The cells were fixed with 4% paraformaldehyde in PBS or 4% paraformaldehyde + 0.1% glutaraldehyde in PBS for 20 min and then washed three times for 10 min with PBS. The fixed cultures were incubated overnight at 4 °C with phospho-TrkB antibody (1:1000), dopamine D1 receptor antibody (1:300; Chemicon), or TrkB antibody (N terminus, 1:300; Upstate) and incubated for 1 h at room temperature with Alexa Fluor® 568 goat anti-rabbit IgG secondary antibody (1:400), Alexa Fluor® 488 goat anti-rabbit IgG secondary antibody (1:200), donkey anti-goat IgG-Cy3 antibody (1:400; Molecular Probes, Eugene, OR), or goat anti-mouse IgG-biotin antibody (1:400; Vector Laboratories, Burlingame, CA). Following mouse IgG-biotin antibody, the cells were incubated with avidin-biotin horseradish peroxidase complexes (VECTASTAIN® Elite ABC kits, Vector Laboratories). The staining was proceeded with diaminobenzidine (0.5 mg/ml in 50 mm Tris, pH 7.5) in the presence of H2O2. The images of immunoreactive neurons were collected with a Nikon fluorescence microscope (ECLIPSE E800) and analyzed using AxioVision (Carl Zeiss, Göttingen, Germany).
Mouse Dopamine D1 Receptor Transfection—The expression vector of mouse D1 receptor (mD1R) was cloned in the mammalian expression vector pcDNA3.1/V5-His©TOPO©TA expression kit (Invitrogen). Expression of D1 receptor was detected by tritium-labeled dopamine binding assay described below.
Human TrkB transfected HEK293 (HEK293-TrkB) cells were prepared as described previously (24) and grown in 12-well plates at a density of 4 × 105 cells/well. The cells were transfected with mD1R plasmid with the calcium phosphate method. After 24 h, the cultured cells were challenged with chemical reagents above. The dopamine binding assay was assessed by incubating HEK293-TrkB cultures for 60 min at 4 °C in Tris Buffer (50 mm Tris, pH 7.4, 120 mm NaCl, 1 mm EDTA, 5 g of glucose, and 2 mm CaCl2) containing 20 nm 3H-dopamine (Amersham Biosciences) and 1 μm dopamine (Sigma). The cells were lysed, and their radioactive content was counted as described previously (21).
Dopamine D1 Receptor Agonist and Antagonist Challenge to Neonatal Rats—Sprague-Dawley rats (4 days old; Charles River Laboratories, Inc.) were housed on a 12-h light-dark cycle with free access to food and water. Different groups of rats were subcutaneously administered at the nape of the neck (25) with SKF38393 (Sigma; 1 mg/kg), SCH23390 (Sigma; 1 mg/kg), or control vehicle (0.25% ascorbic acid in distilled saline). SKF38393 and SCH23390 were dissolved in distilled saline with 0.25% ascorbic acid. The brain tissues of these animals used for immunoprecipitation and immunoblotting were harvested immediately following decapitation and dissected on ice. Peripherally administrated, these compounds penetrate the blood-brain barrier and affect neurochemical makers in the brain (26). The phosphorylation level of dopamine and cyclic AMP-regulated protein (relative molecular mass, 32,000; DARPP32) was increased in striatal tissues from SKF38393-administrated rats and decreased in those from SCH38393-administrated rats (data not shown).
Statistical Analysis—All of the values are presented as the means ± S.D. The pharmacological effects were analyzed using one-way analysis of variance followed by a Bonferroni test or a Mann-Whitney U test to evaluate the differences in immunoblots. A probability level of less than 0.05 was considered to be statistically significant.
RESULTS
Dopaminergic input from the substantia nigra plays an important role in the striatum (22, 27, 28). It has been reported that dopamine D1 receptor signaling can regulate neuronal morphology and expression of functional markers in developing striatal neurons (7, 8). We isolated striatal primary neurons from rat embryonic day 18 embryos and assessed the involvement of neurotrophic signals in the developing neurons.
TrkB, the receptor for BDNF and neurotrophin 4, is expressed in striatum neurons (29). In the striatum, BDNF/TrkB signaling is important in the proliferation, differentiation, and development of medium spiny neurons (30–33). To determine the identity and status of TrkB receptors in striatum neurons, we used a phospho-specific antibody against a C-terminal peptide of TrkB. First, we confirmed the specificity of the anti-phospho-TrkB antibody by a peptide competition assay (see Fig. 2O). Western blot analysis of HEK293-TrkB cells (24) resulted in activated TrkB protein after BDNF treatment. This signal was blocked after preincubation with the phosphorylated TrkB peptide and not with the nonphosphorylated TrkB peptide.
FIGURE 2.

Immunoreactivity of phospho-TrkB receptor induced by D1 receptor agonist in rat striatal neurons. A–N, striatal cultures were treated with control (PBS), SKF38393 (SKF), or BDNF at 37 °C on DIV5. Some dishes were preincubated with K-252a or SCH23390 (SCH) prior to SKF38393 incubation. The cultures were fixed and immunostained with phospho-TrkB antibody (red) and dopamine D1 receptor antibody (green). Note that phospho-TrkB immunoreactivity was detected in 87 ± 11% of D1 receptor positive neurons (data not shown). O, specificity of phospho-TrkB antibody was measured by using HEK293-TrkB cells. To assess specificity of phospho-TrkB antibody, cell lysates (30 μg/each) obtained from HEK293-TrkB cells treated with control (PBS) or BDNF (10 ng/ml) for 5 min were probed with phospho-TrkB antibody as well as the antibody that was preincubated with either a phosphorylated or nonphosphorylated TrkB peptide competitor. WB, Western blotting.
To establish whether Trk receptors are activated by dopamine receptor stimulation in striatum neurons, we prepared primary neuronal cultures from embryonic rat striatum and treated them with the D1 receptor agonist SKF38393 or the D2 receptor agonist quinpirole (34, 35). The neuronal cultures were examined on DIV6 and compared with neurons cultured in the presence of BDNF. Treatment with SKF38393 increased the level of phospho-Trk (Fig. 1A) and phospho-TrkB (Fig. 1B) in striatal neurons. However, the D2 receptor agonist, quinpirole, failed to stimulate the phosphorylation of TrkB (Fig. 1B). Pretreatment with D1 receptor-specific antagonist SCH23390 or tyrosine kinase inhibitor K-252a blocked the increase of TrkB phosphorylation by SKF38393 (Fig. 1B).
FIGURE 1.

Trk receptors phosphorylation by D1 receptor agonist in rat striatal cultures. Neuronal striatal cultures were treated with control (PBS), SKF38393 (SKF;1 μm, 10–180 min), quinpirole (QUN;1 μm, 180min), and BDNF (5 ng/ml, 3 min) at 37 °C on DIV6. Some dishes were preincubated with K-252a (K-252a+SKF) or SCH23390 (SCH+SKF) prior to SKF38393 incubation. A, after immunoprecipitation (IP) with Trk antibody, the cell lysates were subjected to SDS-PAGE and Western blotting (WB) for phosphotyrosine (pY) and Trk. Typical immunoblots (IB) are shown. The protein levels were determined from independent wells (n = 4). *, p < 0.05. The bars indicate S.D. B, some of cell lysates for immunoprecipitation were also subjected to Western blotting. Representative immunoblots are shown. Note that treatment with SKF38393 increased the immunoreactivity of growth-associated protein 43 (a growth cone marker) in this culture (data not shown).
We also examined the activation of Trk signaling molecules, such as Akt, MAPK, phospholipase Cγ, and ARMS (36, 37). After incubation with SKF38393 for 3 h, the phosphorylation levels of several signaling molecules, such as phospholipase Cγ and MAPK, were increased, and they were also inhibited by the pretreatment with SCH23390 or K-252a (Fig. 1B). NSE antibody was used as a neuronal cell marker, and glutamate decarboxylase of 67 kDa (GAD67) was also used as a marker of medium size, spiny GABAergic neurons in striatum (28). The levels of NSE and GAD67 were both constant in our neuronal cultures.
An increase in phospho-TrkB immunoreactivity was also observed in striatal neurons after a 3-h stimulation with SKF38393 (Fig. 2). Phosphorylated TrkB was detected in the D1 receptor-positive neurons. The phospho-TrkB staining was reduced after treatment with SCH23390 or K-252a. These results indicated that TrkB receptors were phosphorylated in striatal neurons specifically by dopamine D1 receptor stimulation, leading to the phosphorylation of TrkB-dependent signaling in vitro.
To determine the effects of D1 receptor activation in vivo, we administered a subcutaneous injection of SKF38393 in postnatal rats (postnatal day 4; Fig. 3). Frontal cortex represents a target region of dopaminergic neurons in the ventral tegmental area. Therefore, frontal cortex and striatum from these animals were dissected after the injection, and the effect of SKF38393 was estimated by measuring activated phospho-TrkB protein level in the dopaminergic target region. In striatal lysates, an increase in phosphorylated TrkB was detected by immunoprecipitation at 3 and 6 h after the injection. No significant increases in phospho-TrkB levels in the frontal cortex were observed. These results suggest that D1 receptor-specific TrkB transactivation occurs in the striatum. We also administered SKF38393 or SCH23390 in postnatal rats (postnatal day 4; Fig. 3B). Three hours after injection, the level of phospho-TrkB was found to be reduced in the striatum of the rats with SCH23390 compared with the control littermates. This experiment confirmed that activation of D1 receptor can regulate TrkB activity in the striatum.
FIGURE 3.

Phospho-TrkB level regulated in the striatum of the rats administrated with D1 receptor agonist and antagonist. Littermate rats were injected with control vehicle (PBS; 0.25% ascorbic acid-saline), SKF38393 (A and B, SKF; 1 mg/kg in 0.25% ascorbic acid-saline) and SCH23390 (B, SCH;1 mg/kg in 0.25% ascorbic acid-saline) on postnatal day 4. A, the effects of D1 receptor stimulation at several time points on striatum and frontal cortex were estimated by determining phospho-TrkB. Duplicate samples are displayed. B, the effects of D1 receptor activation and inhibition after 3 h of administration on striatum were estimated by determining phospho-TrkB. Triplicate samples are displayed. Representative immunoblots (IB) are shown. Quantifications of phospho-TrkB immunoreactivity in striatum by NIH Image are shown. Note that there was no significant reduction of the neuron (NSE) marker in the striatum in this pharmacological paradigm (data not shown. control: 100 ± 12.9%, SKF38393: 100.7 ± 7.1%, SCH23390: 107.4 ± 14.8%, mean ± S.D.). n = 4; *, p < 0.05. The bars indicate S.D. IP, immunoprecipitation.
To confirm the activation of TrkB receptors in a heterologous cell system, we transfected a D1 receptor cDNA construct and assessed the phosphorylation of TrkB by SKF38393 in stably transfected TrkB-HEK293 cells (Fig. 4B). Phospho-TrkB levels were increased by SKF38393 and were reduced by preincubation of K252a or SCH23390, similar to primary neuronal cultures.
FIGURE 4.
Plasmid transfection to HEK293-TrkB cells. A, HEK293-TrkB cells transfected with mouse D1 receptor expression vector (mD1R) or control vector (mock). After 60 min of incubation with 3H-dopamine (3H-DA)-containing buffer, radioactivity contents were determined from independent wells (n = 4). ***. p < 0.001. The bars indicate S.D. B, mD1R transfected HEK293-TrkB cells were treated with control (PBS), SKF38393 (SKF; 1 μm, 180 min), or BDNF (10 ng/ml, 3 min) at 37 °C. Some dishes were preincubated with K-252a ((+)K252a) or SCH23390 ((+)SCH) prior to SKF38393 incubation. The cell lysates were subjected to SDS-PAGE and Western blotting. Typical immunoblots (IB) are shown.
Dopamine can stimulate not only neurons but also non-neuronal cells (38, 39). Our striatal neuronal culture system contains glial cells, ∼10% of total cell numbers (data not shown). Because glial cells increase postnatally, it is therefore questionable whether the TrkB phosphorylation in developing neurons reflects a direct action on these neurons or is mediated through non-neuronal cells reacting to dopamine.
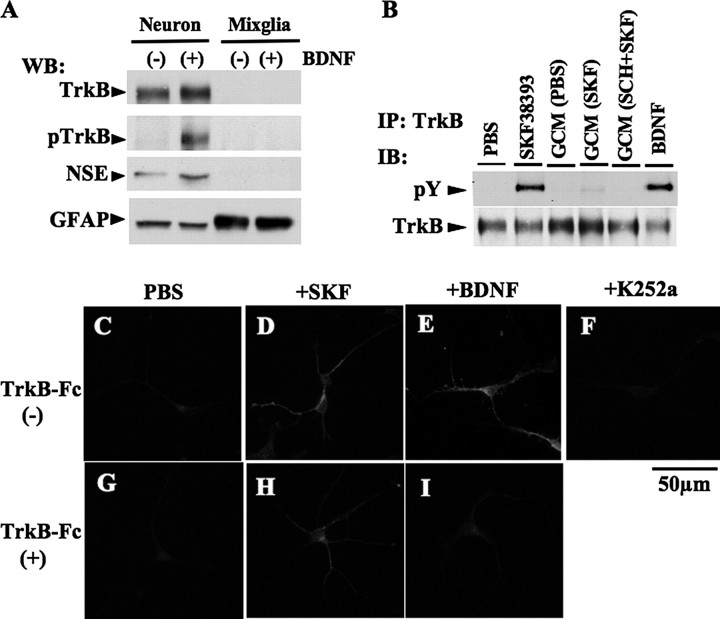
To address the role of glial cells in transactivation, we compared the relative level of full-length TrkB in primary neuronal cultures and primary glial cultures (Fig. 5A). Full-length TrkB was not detectable in glial cell lysates. Next, conditioned medium from primary mixed glia culture was collected after the treatment with control (PBS), SKF38393, or SCH23390 prior to SKF38393 or control (PBS). The neuronal cultures were incubated with glial-conditioned media collected from each treatment, and the levels of phospho-TrkB in the cell lysates were detected by immunoprecipitation (Fig. 5B). No effects were observed in the neuronal cultures from either PBS- or SKF38393-conditioned medium. The results from these experiments suggest that TrkB activation in the striatal neurons by SKF38393 treatment was not mediated by glial cells.
FIGURE 5.
Effects of glial cells or released factors from culture cells on TrkB transactivation in striatal culture. A, striatal neuronal or glial cultures were treated with control (-) or BDNF (+; 10 ng/ml) for 3 min on DIV6. Cell lysates (30 μg each) were subjected to Western blotting (WB). Representative immunoblots (IB) are shown. B, striatal neuronal cultures were treated with conditioned medium from striatal glial cell culture (GCM) on DIV6. Primary glial cells were treated with control (PBS), SKF38393 (SKF; 1 μm, 3 h), or SCH23390 (1 μm, 1 h) prior to SKF38393 (SCH+SKF). After 3 h of incubation, the media were harvested and used as glial condition media for striatal neuronal culture. Neuronal cells were lysed after the treatment with SKF38393, each glial conditioned medium, or BDNF, and then cell samples were immunoprecipitated (IP) with anti-TrkB antibody. Representative immunoblots are shown. C–I, striatal cultures were treated with control (PBS), SKF38393 (+SKF), K-252a and BDNF on DIV6. Some of the cultures were preincubated with TrkB-Fc (10 μg/ml, for 20 min) in 37 °C prior to control (G), SKF38393 (H), or BDNF (I). The cultures were fixed and immunostained with anti-phospho-TrkB antibody.
Another potential explanation for the effect of D1 receptor stimulation is increased production of neurotrophins. Neurotrophins are synthesized as an immature form, which is cleaved and released as a mature form from neurons and non-neuronal cells (40, 41). We tested whether SKF38393-induced TrkB phosphorylation was mediated by the neurotrophin release by applying TrkB-Fc in the culture medium (Fig. 5, C–I). TrkB-Fc contains the extracellular domain of TrkB, which is effective in preventing the activation of native TrkB by sequestering BDNF (42). In striatal cultures, application of TrkB-Fc blocked the increase in phospho-TrkB immunoreactivity by exogenous BDNF but failed to inhibit TrkB phosphorylation after SKF38393 treatment (Fig. 5, H and I). These results provide evidence that the TrkB phosphorylation by D1 receptor stimulation was not mediated by the release of endogenous neurotrophin or from a secondary effect from glial cells.
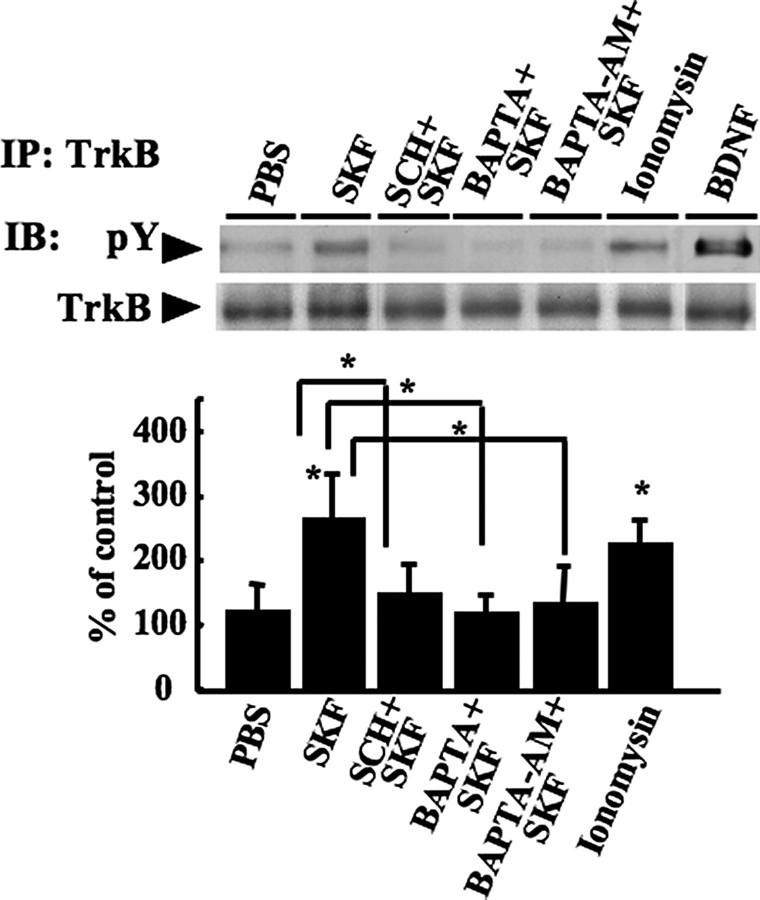
Dopamine receptors can regulate concentration of intracellular calcium ions (Ca2+) by modulating extracellular Ca2+ influx and intracellular Ca2+ pools (11–13). To assess whether Ca2+ contributed to TrkB phosphorylation by the D1 receptor agonist (Fig. 6), we treated striatal cultures with the calcium chelator BAPTA, the membrane-permeable chelator BAPTA-AM, and calcium ionophore ionomycin. The application of ionomycin induced TrkB phosphorylation, in a manner similar to SKF38393. Application of BAPTA or BAPTA-AM abolished the increase in phospho-TrkB levels observed in the presence of SKF38393 alone. In these experiments, we did not detect a significant difference between the effect of BAPTA and BAPTA-AM. Our data suggest that the intracellular concentrations of Ca2+ are regulated by D1 receptor, and increased Ca2+ may be involved in the transactivation of TrkB receptors by SKF38393.
FIGURE 6.
Intracellular Ca2+ regulation is involved in the TrkB transactivation by D1 receptor agonist. Striatal cultures were treated with control, SKF38393 (SKF), ionomycin (10 μm), and BDNF on DIV6. Some of the cultures were preincubated with SCH23390 (SCH+SKF), BAPTA (BAPTA+SKF; 10 μm for 30 min), or BAPTA-AM (BAPTA-AM+SKF; 10 μm for 30 min) in 37 °C prior to SKF38393. After immunoprecipitation (IP), the cell lysates were subjected to SDS-PAGE and Western blotting for phosphotyrosine (pY) and TrkB. Duplicate samples are displayed. Typical immunoblots (IB) are shown. The protein levels were determined from independent wells (n = 4). *, p < 0.05. The bars indicate S.D.
It has been reported that TrkB surface expression is regulated by Ca2+ influx in hippocampal and cortical neurons (42–44). From our results, intracellular calcium is implicated in TrkB transactivation by D1 receptor agonists. To determine the level of surface TrkB expression after the treatment with D1 receptor agonist, we used a cell surface biotinylation assay on primary striatal cultures (Fig. 7, A and B). After a 3-h incubation with SKF38393, striatal neurons were subjected to treatment with NHS-LS-biotin. After immunoprecipitation with avidin-conjugated beads, more biotinylated TrkB was detected on the cell surface (Fig. 7A). Pretreatment with the D1 receptor antagonist, SCH23390, reduced the levels of biotinylated TrkB induced by SKF38393.
FIGURE 7.
Surface TrkB expression regulated by D1 receptor agonist. Striatal cultures were treated with control vehicle (PBS), SKF38393 (SKF), and BDNF on DIV6. Some of the cultures were preincubated with SCH23390 (A, SCH+SKF), K-252a (A), BAPTA (B, BAPTA+SKF), or BAPTA-AM (B, BAPTA-AM+SKF) in 37 °C prior to SKF38393. The cultures were incubated with biotin-PBS for 30 min on ice and then lysed with radioimmune precipitation assay buffer. Half of biotinylated proteins were precipitated by avidin-conjugated beads and then subjected to SDS-PAGE and Western blotting for TrkB. The other samples were used for immunoprecipitation (IP) with TrkB. Duplicate samples are displayed. Typical immunoblots (IB) are shown. Protein levels were determined from independent wells (n = 4). Quantifications of surface biotinylated TrkB levels by NIH Image are shown. *, p < 0.05. The bars indicate S.D. C–H, striatal cultures were treated with control (PBS) and SKF38393 on DIV6. Some of the cultures were preincubated with SCH23390 (SCH+SKF), BAPTA (BAPTA+SKF), or BAPTA-AM (AM+SKF) in 37 °C prior to SKF38393. The cultures were fixed and immunostained with rabbit control IgG (C) or TrkB (N terminus) antibody (D–H) without detergent. Representative pictures are provided.
Similar inhibitory effects were obtained with BAPTA. Pretreatment with BAPTA also lowered the levels of TrkB surface expression. BAPTA-AM also showed the inhibitory effects, but no significant difference was observed compared with SKF38393 treatment (Fig. 7B). In addition to the biotinylation assay, immunocytochemistry using an antibody against the N terminus of TrkB indicated that SKF38393 elevated TrkB surface immunoreactivity. The induction was also blocked by preincubation with SCH23390 or BAPTA. BAPTA-AM pretreatment also inhibited TrkB surface immunoreactivity but not to the same extent as BAPTA (Fig. 7, C–H). These data suggest that the D1 receptor stimulation increases Ca2+ influx and induces the transactivation and cell surface expression of TrkB.
DISCUSSION
Dopamine D1 receptors are linked to the Gs protein and activation of adenylate cyclase. G protein-coupled receptors have various functions from endocrine regulation to higher order behavior. In the central nervous system, GPCRs mediate neuronal functions not only as fast neurotransmitters but also as slow neuromodulators (15). The results presented here indicate that dopamine is capable of transactivating Trk receptors, in a time course similar to other GPCRs, such as adenosine and pituitary adenylate cyclase-activating polypeptide receptors (17, 18). Activation of TrkB through D1 GPCR signaling occurred without involvement of neurotrophins. This suggests that dopamine neurotransmitters are capable of communicating with neurotrophin signaling, which is responsible for many morphological changes in neurons. Trk receptors also regulate the expression and activity of ion channels and neurotransmitter receptors and therefore can modulate synaptic strength and plasticity (36). These studies indicate that mutual regulation between TrkB and dopamine D1 receptor signaling may contribute to normal brain function.
Surface expression levels of receptors are also important in modulating their signaling and function. Du et al. (43) reported that Ca2+ induces TrkB transport to plasma membrane in mature hippocampal neurons. The present data show that dopamine D1 receptor activation increases not only TrkB phosphorylation but also TrkB surface expression through the Ca2+ influx in developing striatal neuron. The findings in this work imply that receptor transactivation events by GPCRs such as D1 receptors may produce different physiological functions and signal transduction mechanisms than by direct ligand-receptor binding.
Dopamine is a predominant neurotransmitter that controls electrophysiological and motor activity. Dopamine has also been linked to neuronal development through its actions on axonal growth and growth cones. For instance, dopamine enhances outgrowth and arborization of processes and growth cones in embryonic striatal neurons (7, 8, 45, 46). Many of these effects have been ascribed to D1 dopamine receptor activity. We observed such enhancement of neuronal development (increasing of neurite extension and growth cone number) by D1 receptor agonist in our culture system (data not shown). These studies suggest that the D1 receptor is crucial for the normal development of striatal neurons during development.
Jung and Bennett (47) observed that acute cocaine treatment increased TrkB mRNA in neonatal striatum. The induction of TrkB mRNA by cocaine was suppressed by SCH23390, a specific antagonist of D1 receptor. Our results did not show a significant enhancement of TrkB protein level in vitro (Fig. 1) or in vivo (Fig. 3) by the D1 receptor-specific agonist SKF38393. Rather we found the transactivation of TrkB was inhibited by SCH23390. It is formally possible that acute stimulation of D1 receptor and subsequent TrkB transactivation might be involved in prolonged transcription and/or translation of TrkB.
Mice lacking D1 receptors displayed growth retardation, but the general anatomy of the brain was normal. The size of the striatum in adult mutant mice was reduced compared with wild type. These mutant mice displayed selective electrophysiological and behavioral alterations (48, 49). These studies indicate that the lack of D1 receptor in the developing brain may alter neurotransmission and behavior in adults. In our experiments, TrkB and phospho-TrkB levels in striatum from D1 receptor null mice were altered compared with wild type mice.4 Therefore, dopamine/dopamine receptor signaling in the knock-out mice differed from wild type animals. However, preliminary analysis made it difficult to establish a causal relationship between dopamine D1 receptor and TrkB signaling. Our results here indicate that dopamine/D1 receptor signaling can contribute to BDNF/TrkB activity. It has been observed that acute BDNF stimulation increased D1R mRNA in vitro, and the D1 receptor mRNA level is decreased in the TrkB null mice (50). Collectively, these studies suggest that BDNF-TrkB and dopamine-D1 receptor signaling are intertwined and involved in the neuronal development in striatum through modulation of neurotrophic responsiveness. However, further work will be needed to fully elucidate the in vivo effects of dopamine on TrkB.
Neurotrophic factors regulate numerous neuronal functions in development and adult life and in response to neuronal injury. As a result, neurotrophins have been implicated in the pathophysiology of a wide variety of neurodegenerative and psychiatric disorders and have been considered to be a therapeutic strategy for neuropsychiatric disorders. Dopaminergic systems also have been studied in relation to Parkinson disease (22, 51) and Huntington disease (52). These parallels suggest that the misbalance in the signaling between neurotrophins and dopamine may be important in neurodegenerative disorders.
Activation of neurotrophin signaling pathways through other receptor systems offers an alternative mechanism of communication in the nervous system. For example, antidepressant agents acting via monoamine G protein-coupled receptors can lead to increased neurotrophin signaling (53). The results with dopamine D1 receptors suggest that agonists of dopamine receptors may be identified with neurotrophic effects for the treatment of neurodegenerative diseases. This approach would involve selective targeting of neurons that express specific GPCRs and trophic factor receptors.
Acknowledgments
We thank all of the members of the Chao laboratory, in particular, Rithwick Rajagopal for phospho-TrkB antibody; Juan Carlos Arevalo, Daniela B. Pereira, and Freddy Jeanneteau for thoughtful advice; and Mercedes Beyna for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants NS21072 and HD23315 (to M. V. C.). This work was also supported by grants from the Core Research for Evolutional Science and Technology from Japan Science and Technology Agency (to H. N. and I. S.) and a grant for the promotion of a Niigata University Research Project (to H. N.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GPCR, G protein-coupled receptor; BDNF, brain-derived neurotrophic factor; DIV, day in vitro; NSE, neuron-specific enolase; MAPK, mitogen-activated protein kinase; PBS, phosphate-buffered saline; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; BAPTA-AM, O,O′-bis(2-aminophenyl)ethylenglycol-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester.
Y. Iwakura, H. Nawa, I. Sora, and M. V. Chao, unpublished data.
References
- 1.Kalivas, P. W. (1993) Brain Res. 18 75-113 [DOI] [PubMed] [Google Scholar]
- 2.Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998) Physiol. Rev. 78 189-225 [DOI] [PubMed] [Google Scholar]
- 3.Bustos, G., Abarca, J., Campusano, J., Bustos, V., Noriega, V., and Aliaga, E. (2004) Brain Res. 47 126-144 [DOI] [PubMed] [Google Scholar]
- 4.Xu, M., Hu, X. T., Cooper, D. C., Moratalla, R., Graybiel, A. M., White, F. J., and Tonegawa, S. (1994) Cell 79 945-955 [DOI] [PubMed] [Google Scholar]
- 5.Centonze, D., Grande, C., Saulle, E., Martin, A. B., Gubellini, P., Pavon, N., Pisani, A., Bernardi, G., Moratalla, R., and Calabresi, P. (2003) J. Nuerosci. 23 8506-8512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtani, N., Goto, T., Waeber, C., and Bhide, P. G. (2003) J. Neurosci. 23 2840-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt, U., Beyer, C., Oestreicher, A. B., Reisert, I., Schilling, K., and Pilgrim, C. (1996) Neuroscience 74 453-460 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt, U., Pilgrim, C., and Beyer, C. (1998) Mol. Cell Neurosci. 11 9-18 [DOI] [PubMed] [Google Scholar]
- 9.Jassen, A. K., Yang, H., Miller, G. M., Calder, E., and Madras, B. K. (2006) Mol. Pharmacol. 70 71-77 [DOI] [PubMed] [Google Scholar]
- 10.Jackson, D. M., and Westlind-Danielsson, A. (1994) Pharmacol. Ther. 64 291-370 [DOI] [PubMed] [Google Scholar]
- 11.Surmeier, D. J., Bargas, J., Hemmings, H. C., Nairn, A. C., and Greengard, P. (1995) Neuron 14 385-397 [DOI] [PubMed] [Google Scholar]
- 12.Hernández-López, S., Bargas, J., Surmeier, D. J., Reyes, A., and Galarraga, E. (1997) J. Neurosci. 17 3334-3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ming, Y., Zhang, H., Long, L., Wang, F., Chen, J., and Zhen, X. (2006) J. Neurochem. 98 1316-1323 [DOI] [PubMed] [Google Scholar]
- 14.Bloch, B., Bernard, V., and Dumartin, B. (2003) Biol. Cell 95 477-488 [DOI] [PubMed] [Google Scholar]
- 15.Gainetdinov, R. R., Premont, R. T., Bohn, L. M., Lefkowitz, R. J., and Caron, M. G. (2004) Annu. Rev. Neurosci. 27 107-144 [DOI] [PubMed] [Google Scholar]
- 16.Huang, E. J., and Reichardt, L. F. (2003) Annu. Rev. Biochem. 72 609-642 [DOI] [PubMed] [Google Scholar]
- 17.Lee, F. S., and Chao, M. V. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 3555-3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajagopal, R., Chen, Z. Y., Lee, F. S., and Chao, M. V. (2004) J. Neurosci. 24 6650-6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berghuis, P., Dobszay, M. B., Wang, X., Spano, S., Ledda, F., Sousa, K. M., Schulte, G., Ernfors, P., Mackie, K., Paratcha, G., Hurd, Y. L., and Harkany, T. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 19115-19120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiese, S., Jablonka, S., Holtmann, B., Orel, N., Rajagopal, R., Chao, M. V., and Sendtner, M. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 17210-17215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwakura, Y., Nagano, T., Kawamura, M., Horikawa, H., Ibaraki, K., Takei, N., and Nawa, H. (2001) J. Biol. Chem. 276 40025-40032 [DOI] [PubMed] [Google Scholar]
- 22.Iwakura, Y., Piao, Y. S., Mizuno, M., Takei, N., Kakita, A., Takahashi, H., and Nawa, H. (2005) J. Neurochem. 93 974-983 [DOI] [PubMed] [Google Scholar]
- 23.Arévalo, J. C., Waite, J., Rajagopal, R., Beyna, M., Chen, Z. Y., Lee, F. S., and Chao, M. V. (2006) Neuron 50 549-559 [DOI] [PubMed] [Google Scholar]
- 24.Narisawa-Saito, M., Iwakura, Y., Kawamura, M., Araki, K., Kozaki, S., Takei, N., and Nawa, H. (2002) J. Biol. Chem. 277 40901-40910 [DOI] [PubMed] [Google Scholar]
- 25.Yokomaku, D., Jourdi, H., Kakita, A., Nagano, T., Takahashi, H., Takei, N., and Nawa, H. (2005) Neuroscience 136 1037-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis, R. M., Levari, I., Ihrig, B., and Zigmond, M. J. (1990) J. Neurochem. 55 1071-1074 [DOI] [PubMed] [Google Scholar]
- 27.Ingham, C. A., Hood, S. H., van Maldegem, B., Weenink, A., and Arbuthnott, G. W. (1993) Exp. Brain Res. 93 17-27 [DOI] [PubMed] [Google Scholar]
- 28.Goldberg, M. S., Pisani, A., Haburcak, M., Vortherms, T. A., Kitada, T., Costa, C., Tong, Y., Martella, G., Tscherter, A., Martins, A., Bernardi, G., Roth, B. L., Pothos, E. N., Calabresi, P., and Shen, J. (2005) Neuron 45 489-496 [DOI] [PubMed] [Google Scholar]
- 29.Altar, C. A., Siuciak, J. A., Wright, P., Ip, N. Y., Lindsay, R. M., and Wiegand, S. J. (1994) Eur. J. Neurosci. 6 1389-1405 [DOI] [PubMed] [Google Scholar]
- 30.Mizuno, K., Carnahan, J., and Nawa, H. (1994) Dev. Biol. 165 243-256 [DOI] [PubMed] [Google Scholar]
- 31.Ivkovic, S., Polonskaia, O., Fariñas, I., and Ehrlich, M. E. (1997) Neuroscience 79 509-516 [DOI] [PubMed] [Google Scholar]
- 32.Ivkovic, S., and Ehrlich, M. E. (1999) J. Neurosci. 19 5409-5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benraiss, A., Chmielnicki, E., Lerner, K., Roh, D., and Goldman, S. A. (2001) J. Neurosci. 21 6718-6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibley, D. R., Leff, S. E., and Creese, I. (1982) Life Sci. 31 637-645 [DOI] [PubMed] [Google Scholar]
- 35.Tsuruta, K., Frey, E. A., Grewe, C. W., Cote, T. E., Eskay, R. L., and Kebabian, J. W. (1981) Nature 292 463-465 [DOI] [PubMed] [Google Scholar]
- 36.Chao, M. V. (2003) Nat. Rev. Neurosci. 4 299-309 [DOI] [PubMed] [Google Scholar]
- 37.Arévalo, J. C., Yano, H., Teng, K. K., and Chao, M. V. (2004) EMBO J. 23 2358-2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuss, B., and Unsicker, K. (2001) Mol. Cell Neurosci. 18 197-209 [DOI] [PubMed] [Google Scholar]
- 39.Färber, K., Pannasch, U., and Kettenmann, H. (2005) Mol. Cell Neurosci. 29 128-138 [DOI] [PubMed] [Google Scholar]
- 40.Edwards, R. H., Selby, M. J., Garcia, P. D., and Rutter, W. J. (1988) J. Biol. Chem. 263 6810-6815 [PubMed] [Google Scholar]
- 41.Lessmann, V., Gottmann, K., and Malcangio, M. (2003) Prog. Neurobiol. 69 341-374 [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Franke, A., Wilkinson, G. A., Kruttgen, A., Hu, M., Munro, E., Hanson, M. G., Reichardt, L. F., and Barres, B. A. (1998) Neuron 21 681-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du, J., Feng, L., Yang, F., and Lu, B. (2000) J. Cell Biol. 150 1423-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingsbury, T. J., Murray, P. D., Bambrick, L. L., and Krueger, B. K. (2003) J. Biol. Chem. 278 40744-40748 [DOI] [PubMed] [Google Scholar]
- 45.Hess, E. J., Battaglia, G., Norman, A. B., Iorio, L. C., and Creese, I. (1986) Eur. J. Pharmacol. 121 31-38 [DOI] [PubMed] [Google Scholar]
- 46.Berg, M. M., Sternberg, D. W., Parada, L. F., and Chao, M. V. (1992) J. Biol. Chem. 267 13-16 [PubMed] [Google Scholar]
- 47.Jung, A. B., and Bennett, J. P., Jr. (1996) Brain Res. Dev. Brain Res. 94 133-143 [DOI] [PubMed] [Google Scholar]
- 48.Drago, J., Gerfin, C. R., Lachowicz, J. E., Steiner, H., Hokkon, T. R., Love, P. E., Ooi, G. T., Grinberg, A., Lee, E. J., Huang, S. P., Bartlett, P. F., Jose, P. A., Sibley, D. R., and Westphal, H. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 12564-12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, M., Moratalla, R., Gold, L. H., Hiroi, N., Koob, G. F., Graybiel, A. M., and Tonegawa, S. (1994) Cell 79 729-742 [DOI] [PubMed] [Google Scholar]
- 50.Do, T., Kerr, B., and Kuzhikandathil, E. V. (2007) J. Neurochem. 100 416-428 [DOI] [PubMed] [Google Scholar]
- 51.Mayeux, R. (2003) Annu. Rev. Neurosci. 26 81-104 [DOI] [PubMed] [Google Scholar]
- 52.Pineda, J. R., Canals, J. M., Bosch, M., Adell, A., Mengod, G., Artigas, F., Ernfors, P., and Alberch, J. (2005) J. Neurochem. 93 1057-1058 [DOI] [PubMed] [Google Scholar]
- 53.Duman, R. S., Heninger, G. R., and Nestler, E. J. (1997) Arch. Gen. Psychiatry. 54 597-606 [DOI] [PubMed] [Google Scholar]