Abstract
EphA2 is a member of the Eph family of receptor tyrosine kinases. EphA2 mediates cell-cell communication and plays critical roles in a number of physiological and pathologic responses. We have previously shown that EphA2 is a key regulator of tumor angiogenesis and that tyrosine phosphorylation regulates EphA2 signaling. To understand the role of EphA2 phosphorylation, we have mapped phosphorylated tyrosines within the intracellular region of EphA2 by a combination of mass spectrometry analysis and phosphopeptide mapping using two-dimensional chromatography in conjunction with site-directed mutagenesis. The function of these phosphorylated tyrosine residues was assessed by mutational analysis using EphA2-null endothelial cells reconstituted with EphA2 tyrosine-to-phenylalanine or tyrosine-to-glutamic acid substitution mutants. Phosphorylated Tyr587 and Tyr593 bind to Vav2 and Vav3 guanine nucleotide exchange factors, whereas Tyr(P)734 binds to the p85 regulatory subunit of phosphatidylinositol 3-kinase. Mutations that uncouple EphA2 with Vav guanine nucleotide exchange factors or p85 are defective in Rac1 activation and cell migration. Finally, EphA2 mutations in the juxtamembrane region (Y587F, Y593F, Y587E/Y593E), kinase domain (Y734F), or SAM domain (Y929F) inhibited ephrin-A1-induced vascular assembly. In addition, EphA2-null endothelial cells reconstituted with these mutants were unable to incorporate into tumor vasculature, suggesting a critical role of these phosphorylation tyrosine residues in transducing EphA2 signaling in vascular endothelial cells during tumor angiogenesis.
The Eph receptors belong to a large family of receptor tyrosine kinases that regulate a variety of physiological processes during development and contribute to the pathogenesis of diseases such as cancer (1, 2). One of the key events important both in embryogenesis and pathogenesis in adult organisms is angiogenesis, the process by which new blood vessels are formed from preexisting vasculature. On the basis of sequence homology and binding affinity, the Eph receptors are divided into two subclasses. EphA receptors bind preferentially to the glycosylphosphatidylinositol-linked ephrin-A ligands, whereas EphB receptors bind preferentially to the transmembrane ephrin-B ligands (3). Both class A and class B Eph receptors have been implicated in regulation of vascular remodeling and angiogenesis. Targeted disruption of several class B receptor tyrosine kinases and ephrin-B ligands resulted in defects in angiogenic remodeling of the rudimentary embryonic vasculature (4-7). Manipulation of the level of one receptor, EphB4, in tumor cells also affected tumor angiogenesis in adult animals (8, 9). In the A class, ephrin-A1 stimulates endothelial cell migration and assembly in culture (10, 11) and induces corneal angiogenesis in vivo (12, 13). More recently, Eph receptors have been detected in tumor blood vessel endothelial cells (1, 5). Inhibition of class A Eph receptor signaling by soluble EphA2-Fc or EphA3-Fc receptors decreased tumor volume, tumor angiogenesis, and metastatic progression in vivo (14-16). A main target of soluble EphA receptors appears to be EphA2, since EphA2-deficient endothelial cells fail to migrate and assemble in vitro (17), and loss of EphA2 receptor resulted in impaired tumor growth and metastasis in vivo (18).
The binding of ephrin ligands to Eph receptors induces the transphosphorylation of the cytoplasmic domains and initiates kinase activity. Extensive tyrosine phosphorylation of the activated Eph receptor is not only induced by auto/trans-phosphorylation but is also elicited by receptor-associated protein-tyrosine kinases such as Src family kinases (2). Many phosphorylated tyrosine residues in the EphB receptors and ephrin-B ligands in neuronal cells/tissues have been mapped by both phosphopeptide mapping using two-dimensional chromatography, and by matrix-assisted laser desorption/ionization mass spectrometry (19-21). Several tyrosine phosphorylation sites in EphA3 and EphA4 have also been identified by mutational analysis on sites homologous to those in EphB receptors (21, 22). However, since these phosphorylated tyrosine residues are not mapped in endothelial cells, their role in signal transduction leading to angiogenic responses is not clear. Moreover, phosphorylated tyrosine residues have not been mapped in EphA2, a major EphA receptor that is critical in mediating tumor angiogenesis.
We have previously shown that activation of the EphA2 receptor in endothelial cells recruits Vav GEFs,2 resulting in up-regulation of GTP-bound activated Rac1 GTPase and endothelial cell migration (23). The Vav GEF/Rac1 pathway appears to be regulated by PI 3-kinase, since PI 3-kinase-specific inhibitors wortmannin and LY294002 or a dominant negative p85 subunit of PI 3-kinase blocks ephrin-A1-induced Rac1 activation and endothelial cell migration (17). Since the SH2 domains of both Vav GEFs and p85 subunit of the PI 3-kinase are capable of binding to phosphorylated EphA2 receptor (23, 24), we sought to identify critical phosphorylated tyrosine residues that mediate the recruitment of Vav GEFs and p85. As a first step, we have used a combination of mass spectrometry analysis and traditional phosphopeptide mapping to identify the phosphorylated tyrosine residues within the EphA2 receptor. Four phosphorylated tyrosine residues in the cytoplasmic domain of the EphA2 receptor were identified. Changing three of these sites to phenylalanine or glutamic acid resulted in an EphA2 mutant that could not be phosphorylated, failed to interact with p85 or Vav GEFs, and was unable to rescue defects in endothelial assembly in EphA2-deficient cells in vitro and in vivo. Our results suggest that phosphorylation of Tyr587/Tyr593 and Tyr734 is critical in recruitment of Vav and p85, respectively. Phosphorylation of these tyrosines is also essential in activation of Rac1 GTPase and promoting angiogenic responses and tumor neovascularization.
EXPERIMENTAL PROCEDURES
Plasmids, Antibodies, and Reagents—Antibodies used for immunoblot include anti-EphA2 (1:1000, Upstate Biotechnology), anti-phosphotyrosine (1:250; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-tubulin (1:1000, Sigma), and anti-Rac1 and anti-Cdc42 (1:1000; Transduction Laboratories). Immunoprecipitation of EphA2 from cell lysates was performed with anti-EphA2 antibody (2 μg; Upstate Biotechnology), and p85 was immunoprecipitated by anti-FLAG (M2)-agarose beads (Sigma). Recombinant ephrin-A1-Fc proteins were purchased from R&D Systems (Minneapolis, MN). Growth factor-reduced Matrigel was purchased from BD Biosciences. Transient transfection was performed using Lipofectamine 2000 (Invitrogen).
EphA2 mutations were generated by PCR amplification using EphA2-specific primers containing tyrosine to phenylalanine or glutamic acid mutations. The fragments were digested with AgeI and BsiWI (for tyrosine mutations 593-846) and BamHI and BlpI (for tyrosine mutations 921-959) and ligated into the digested plasmids pcDNA3.0-EphA2 and LZRS-EphA2. All mutations were verified by DNA sequencing.
LC-MS Analysis—LC-MS was performed by the Proteomics Laboratory in the Vanderbilt Mass Spectrometry Research Center. Resolved mouse EphA2 was excised from SDS-polyacrylamide gels for in-gel digestion with trypsin (25). The resulting peptides were separated by reverse phase high pressure liquid chromatography that is coupled directly with automatic tandem MS (LC-MS) using a ThermoFinnigan LTQ ion trap mass spectrometer equipped with a Thermo surveyor autosampler and Thermo Surveyor HPLC pump, nanospray source, and Xcalibur 1.4 instrument control. HPLC separation of the tryptic peptides was achieved with a 100 mm × 11-cm C-18 capillary column (Monitor C18, 5 μm, 100 Å; Column Engineering), at a 0.7 μl min-1 flow rate. Solvent A was H2O with 0.1% formic acid, and solvent B was acetonitrile containing 0.1% formic acid. The gradient program was as follows: 0-3 min, linear gradient from 0-5% B; 3-5 min, 5% B; 5-50 min, linear gradient to 50% B; 50-52 min, linear gradient to 80% B; 52-55 min, linear gradient to 90% B; 55-56 min, 90% B in solvent A. MS/MS scans were acquired using an isolation width of 2 m/z, an activation time of 30 ms, and activation Q of 0.250 and 30% normalized collision energy using one microscan and an ion time of 100 for each scan. The mass spectrometer was tuned prior to analysis using the synthetic peptide TpepK (AVAGKAGAR). Typical tune parameters were as follows: spray voltage of 1.8 kV, a capillary temperature of 160 °C, a capillary voltage of 60 V, and tube lens 120 V. Initial analysis was performed using data-dependent scanning in which one full MS spectrum, using a full mass range of 400-2000 atomic mass units, was followed by three MS/MS spectra. Incorporated into the method was a data-dependent scan for the neutral loss of phosphoric acid or phosphate (-98, -80), such that if these masses were found, an MS/MS/MS of the neutral loss ion was performed. Peptides were identified using a cluster-compatible version of the SEQUEST algorithm (26, 27), using a mouse subset of proteins from the nonredundant data base from NCBI downloaded in January, 2004 containing 90, 197 sequences. Sequest searches were done on a high speed, multiprocessor Linux cluster in the Advanced Computing Center for Research. In addition to using the SEQUEST algorithm to search for phosphorylation on serines, threonines, or tyrosines, the data were also analyzed using the Pmod algorithm (28). All possible modified peptides were verified by manual inspection of the spectra.
Endothelial Cell Culture and Retroviral Infection—Wild-type or EphA2-deficient primary murine pulmonary microvascular endothelial cells were isolated from 1-3-month-old mice derived from H-2Kb-tsA58 transgenic “Immorto-mouse” background (17, 29). These cells were grown at 33 °C in EGM-2 medium supplemented with interferon-γ (10 ng/ml), a permissive condition that allows the expression of SV40 T-antigen (TAg). The EphA2-deficient endothelial cells were infected with LZRS retroviruses co-expressing IRES-EphA2 (wild-type or mutant)-green fluorescent protein and sorted by a fluorescence-activated cell sorter for comparable EphA2 receptor levels. Cells were placed at physiologic temperature (37 °C) for 4 days to down-regulate thermolabile TAg before experiments.
Phosphopeptide Mapping by Two-dimensional Chromatography—EphA2-null murine pulmonary microvascular endothelial cells reconstituted with either wild-type or mutant EphA2 were stimulated with ephrin-A1 for 15 min. Cells were lysed and EphA2 was immunoprecipitated and phosphorylated in the presence of [γ-32P]ATP, as described under “Immunoprecipitation, Western Blot Analysis, and Kinase Assay.” Immunoprecipitates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane. Polyvinylidene difluoride membrane containing 32P-labeled EphA2 receptor was excised, and proteins were digested in membrane with 1 mg/ml l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin. The resulting peptide mixture was resolved in two dimensions on 20 cm × 20-cm thin layer cellulose plates by electrophoresis followed by ascending chromatography. Electrophoresis was performed at pH 1.9 in 10:1:189 acetic acid/pyridine/water for 3 h at 250 V with ∼10 p.s.i. of pressure. Ascending chromatography was carried out in 625:19:48:29: 279 isobutyric acid/n-butanol/pyridine/acetic acid/water for 11 h or until the buffer was about 1 cm from the top of the TLC plate. The plates were dried and subjected to autoradiography overnight at -70 °C with an intensifying screen.
Immunoprecipitation, Western Blot Analysis, and Kinase Assay—Co-immunoprecipitation of EphA2 and Vav2/Vav3 were performed as described (23). For co-immunoprecipitation of EphA2 with p85, COS7 cells were co-transfected with 1 μg each of EphA2 and FLAG-tagged p85 per well in a 6-well dish using Lipofectamine 2000. p85 was immunoprecipitated by FLAG-agarose beads (20 μl of beads/ml of lysate; Sigma). The resulting proteins were resolved on SDS-PAGE and Western blotted using anti-EphA2 (D7; 1:1000).
Kinase assays using EphA2 as substrate were performed as described previously (30). Kinase assays were also performed using a single exogenous substrate, biotinylated poly(Glu-Tyr) (1 μg/reaction) according to the manufacturer's instructions (Millipore). Briefly, following the kinase reaction, the samples were denatured by heating for 5 min. Streptavidin-agarose beads were added to the supernatant to precipitate substrates. Tyrosine phosphorylation of substrates was quantified using a scintillation counter.
Vascular Assembly Assay—In vitro vascular assembly assays were performed as described previously (17). Briefly, 12-well plates were coated with 100 μl of growth factor reduced Matrigel (BD Biosciences). After 24-h starvation in Opti-MEM, 25,000 cells were plated in wells in the presence or absence of ephrin-A1 (1.5 μg/ml; R&D Systems) and photographed after 9 h. Images were acquired on an Olympus CK40 inverted microscope through an Optronics DEI-750C CCD video camera using Scion Image version 1.62c capture software. The degree of assembly was quantified by measuring branch length, the distance from branching point to the tip of assembled cells. Only assembled cells consisting of at least three cells were measured. The branch length in assembled endothelial cell networks was expressed as arbitrary units per ×10 field in four random fields from each well, with triplicate samples per condition, using Scion Image version 1.62c software for analysis.
Guanine Nucleotide Exchange Assays—For Rac1 and Cdc42 activation assays, cells were serum-starved for 24 h in Opti-MEM medium, followed by stimulation with ephrin-A1 (1 μg/ml). Lysates were prepared and incubated with Pak-1 binding domain-GST beads according to the manufacturer's instructions (Upstate Biotechnology). Proteins were then separated by SDS-PAGE electrophoresis and transferred to a nitrocellulose filter. Activated Rac1 and Cdc42 (or total Rac1 and Cdc42 in lysates) were detected by immunoblotting using anti-Rac1 or anti-Cdc42 antibodies (BD Transduction Laboratories). Relative levels of GTP-bound Rac1 and Cdc42 were quantified by densitomitry using Scion Image version 1.62c software analysis.
Transwell Migration Assay—Endothelial cells were serum-starved for 24 h in Opti-MEM medium. Transwells were coated with growth factor-reduced Matrigel (BD Biosciences; 1:20 dilution with Opti-MEM) for 30 min and blocked with 1% bovine serum albumin solution for an additional 30 min. 200,000 cells were plated in the upper chamber of the transwells, and 600 ml of Opti-MEM medium containing ephrin-A1-Fc (1 μg/ml) was added to the lower chamber. After 5 h, cells were fixed and stained with crystal violet to visualize endothelial cells. Cells that have migrated to the lower surface of transwell filters were counted in four random fields from each well, with triplicate samples per condition.
Co-transplantation of Tumor Cells and Endothelial Cells—Tumor-endothelial cell co-transplantation experiments were performed as described previously (18). Briefly, EphA2-null endothelial cells reconstituted with either wild-type or mutant EphA2 were transduced with 1 × 108 plaque-forming units/ml Ad-β-galactosidase adenovirus. 4T1 tumor cells (50,000 cells) and Ad-β-galactosidase-transduced endothelial cells (5 × 105) were resuspended in 300 μl of growth factor-reduced Matrigel and injected into the subcutaneous dorsal flank of 10-week-old BALB/c nude female mice. Tumors were collected 7 days post-transplantation, and tumor volume was assessed using the following formula: volume = length × width2 × 0.52. Cryosections were processed first for X-gal staining and then subjected to CD31 immunohistochemistry as described previously (18). Data are a representation of eight independent tumors/conditions from two independent experiments.
RESULTS
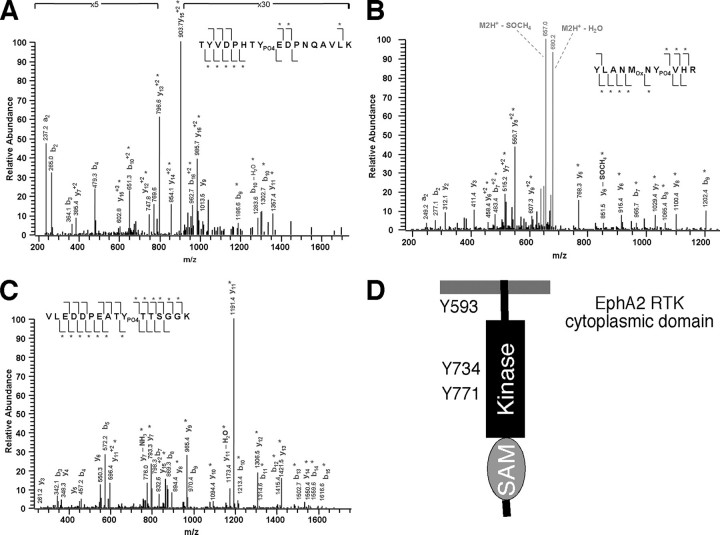
Mapping Tyrosine Phosphorylation Sites in EphA2 Receptor—To identify the phosphorylated tyrosine residues in the cytoplasmic domain of EphA2 receptor induced upon binding to ephrin-A1 ligand, we initially expressed EphA2 in COS7 cells. Immunoprecipitated EphA2 proteins were digested with trypsin and subjected to LC-MS mass spectrometric analysis. Greater than 50% of the tryptic peptides were not detected and were therefore not analyzed. Among the remaining tryptic peptides analyzed, three phosphorylated peptides were identified that contained Tyr593 in the juxtamembrane domain as well as Tyr734 and Tyr771 in the kinase domain (Fig. 1 and Table 1).
FIGURE 1.
In vivo tyrosine phosphorylation sites of EphA2 in transfected COS7 cells. A-C, LC-MS mass spectra of tyrosine-phosphorylated tryptic peptides from immunoprecipitated EphA2. Tyrosine-phosphorylated peptides were isolated from the resulting peptide mixture and purified by reverse phase chromatography. Peaks corresponding to tyrosine-phosphorylated peptides of EphA2 are denoted by their masses and sequences. A, triply charged peptide with m/z 691.4. B, doubly charged peptide with m/z 688.69. C, doubly charged peptide with m/z 882.14. D, schematic diagram of phosphorylated tyrosine residues in the cytoplasmic domains of the EphA2 receptor.
TABLE 1.
Tryptic peptides from in vivo phosphorylated EphA2 identified by mass spectrometry
PO4, phosphorylated tyrosine.
| Tyrosine | Masscalca | Massmeasuredb | Δc | Peptide |
|---|---|---|---|---|
| Tyr593 | 2068.9 | 2071.1 | 2.2 | TYVDPHTYPO4EDPNQAVLKd |
| Tyr734 | 1375.6 | 1375.4 | 0.2 | YLANMOXNYPO4VHR |
| Tyr771 | 1761.7 | 1762.3 | 0.6 | VLEDDPEATYPO4TTSGGK |
Masscalc, calculated mass.
Massmeasured, measured mass.
Δ, difference between measured mass and calculated mass.
OX, oxidized methionine.
To verify phosphorylation sites mapped by mass spectrometry and to identify additional sites not covered by mass spectrometric analysis, we performed phosphopeptide mapping by two-dimensional chromatography in conjunction with site-directed mutagenesis. We chose to use immortalized EphA2-null and wild-type control endothelial cell lines for our analysis, since the EphA2-null background facilitates mutational analysis and subsequent functional assays. These endothelial cells were isolated from EphA2-deficient mice that were bred into the H-2Kb-tsA58 transgenic Immorto-mouse background (29). These Immorto-mice harbor a temperature-sensitive SV40 TAg cassette driven by the mouse major histocompatibility complex H-2Kb promoter, which permits expression in a wide array of tissues. In addition, the promoter is responsive to interferon-γ, permitting elevated expression of the TAg in cells derived from these mice when cultured at 33 °C in the presence of interferon-γ. Once cells are placed at physiologic temperature (37 °C), protein levels of the thermolabile TAg are down-regulated, and cells are restored to a nontransformed state over the course of several days (29).
Wild-type and a panel of Tyr to Phe mutant EphA2 constructs were stably expressed in endothelial cells via retroviral transduction using the LZRS retroviral system (30). In vitro kinase assays using an exogenous substrate revealed that Y593E, Y587E/Y593E, Y734F and Y771F mutations do not affect kinase activity significantly. However, Y587F and Y929F inhibited and Y593F abolished EphA2 kinase activity (Fig. 2A). Tyr587/Tyr593 and Tyr771 appeared to be major tyrosine phosphorylation sites in the EphA2 receptor, since phosphorylation of EphA2 was markedly reduced in Y587E/Y593E and Y771F mutants (Fig. 2B), despite the observation that these mutants retained kinase activity.
FIGURE 2.
Wild-type and mutant EphA2 kinase activity. A, an in vitro kinase assay was performed on EphA2 immunoprecipitated from EphA2-null endothelial cells reconstituted with wild-type or mutant EphA2 via LZRS retroviral transduction. EphA2 kinase activity was measured by its ability to phosphorylate the synthetic substrate, poly(Glu-Tyr) (4:1). B, phosphorylated EphA2 (p-EphA2) levels were assayed in EphA2-null endothelial cells reconstituted with wild type or mutant EphA2. EphA2 was immunoprecipitated (IP) by an anti-EphA2 antibody, and tyrosine phosphorylation was detected by a mixture of anti-Tyr(P)20 and anti-Tyr(P)99 antibodies. IB, immunoblot.
Phosphopeptide mapping by two-dimensional chromatography detected five distinct phosphopeptides in activated wild-type EphA2 (Fig. 3, Experiment #1). To identify the phosphorylated tyrosines within the tryptic peptides, the phosphorylated tyrosines identified by mass spectrometry analysis or those tyrosine residues that were not covered were mutated to phenylalanine. These include tyrosine residues in the juxtamembrane region (Y587F, Y593F), kinase domain (Y685F, Y693F, Y734F, Y771F, Y802F, Y812F, Y816F, and Y846F), and the carboxyl terminal SAM domain (Y921F, Y929F, and Y959F). Because the Y593F could not be analyzed due to defective kinase activity resulting in insufficient γ-32P incorporation, a tyrosine to glutamic acid mutant, Y593E, that retained kinase activity was used for further analysis. Tryptic phosphopeptide maps of wild-type microvascular endothelial cells were similar to those EphA2-null cells reconstituted with wild-type EphA2 receptor (Fig. 3, Experiment #1). Each of the EphA2 mutants was deficient in certain γ-32P-labeled phosphopeptides. The Y587F mutant lacks two major phosphopeptides (a and b). Phosphopeptide c was absent in the Y593E mutant, and phosphopeptide d was absent in the Y771F mutant. Phosphorylation of Tyr734 was identified in a separate experiment when the first dimension chromatography was performed in the reverse direction (Fig. 3, Experiment #2). Taken together, these results suggest that Tyr587, Tyr593, Tyr771, and Tyr734 are likely to be autophospho-rylated in vascular endothelial cells.
FIGURE 3.
Tryptic phosphopeptide maps of wild-type (WT) and mutant (knock-out; KO) forms of EphA2. Wild-type and mutant forms of EphA2 were expressed in EphA2-null endothelial cells via LZRS retroviral transduction. EphA2 receptors were immunoprecipitated, phosphorylated in the presence of [γ-32P] ATP, and resolved by SDS-PAGE. Radioactive bands representing phosphorylated forms of EphA2 were excised, digested with trypsin, and subjected to two-dimensional chromatography. Four peptides were detected (labeled a-d) in both wild-type and EphA2-null reconstituted with wild-type EphA2 in Experiment 1. An additional phosphopeptide (labeled e) was identified in Experiment 2 when the first dimension chromatography was performed in the reverse direction. Peptides containing identified phosphorylated tyrosines are indicated on the left in two schematic representations, one for each set of experiments.
Vav GEFs Binds to Tyr(P)587/Tyr(P)593 in the Juxtamembrane Region, and p85 Interacts with Tyr(P)734 in the EphA2 Kinase Domain—We have previously shown that guanine nucleotide exchange factors Vav2 and Vav3 are recruited to phosphorylated EphA2 receptor, and the binding is significantly reduced in Y587F/Y593F double mutants (23). To assess which phosphorylated tyrosine residue or whether both Tyr(P) sites in the juxtamembrane region of the EphA2 is/are required for interaction with Vav proteins, we performed a series of co-immunoprecipitation experiments coupled with Western blot analysis. As shown in Fig. 4A, mutation at either Tyr587 or Tyr593 inhibited binding of EphA2 receptor to Vav2 and Vav3 exchange factors, suggesting that both sites are required for optimal binding to Vav GEFs. Interestingly, Tyr929 in the SAM domain also appears to affect binding to Vav3 but not Vav2 GEF. Since phosphorylation of Tyr929 was not detected by in vitro kinase assay, this site might be phosphorylated by another tyrosine kinase in vivo.
FIGURE 4.
Mapping of Vav and p85 binding sites in EphA2 receptor. A, the wild-type or mutant EphA2 and Vav2 or Vav3 were co-expressed in the COS7 cells. The Vav2 or Vav3 proteins were immunoprecipitated (IP) and Western blotted using antibodies against EphA2. The blots were stripped and reprobed using antibodies against Vav2 or Vav3 for confirmation of equal loading. B, the wild-type or mutant EphA2 and FLAG-tagged p85 were co-expressed in the COS7 cells. The p85 proteins were immunoprecipitated and Western blotted using antibodies against EphA2. The blots were stripped and reprobed by anti-FLAG for equal loading. EE, a double Tyr to Glu mutation (Y587E/Y593E) in the juxtamembrane domain. C, wild-type or mutant Vav2, Vav3, or p85 were co-expressed with EphA2 in COS7 cells. EphA2 or Vav proteins were immunoprecipitated by appropriate antibodies and Western blotted by antibodies against p85 and EphA2, respectively.
In addition to binding to Vav GEFs, we and others have shown that activated EphA2 receptor also recruits the p85 subunit of the PI 3-kinase, and PI 3-kinase activity is required for ephrin-A1-induced Rac1 GTPase activation and endothelial cell migration (17, 24). However, although the SH2 domain of the p85 was shown to interact with the kinase domain of the EphA2 receptor (24), the precise phosphotyrosine residue that mediates this interaction is unknown. Thus, a panel of EphA2 mutants containing Tyr to Phe mutations was tested for the ability to bind to p85. As shown in Fig. 4B, although p85 binds to Y587F, Y593E, Y587E/A593E, and Y771F as well as wild-type EphA2, it fails to bind to Y734F and Y929F, suggesting that p85 interacts with phosphorylated Tyr734 in the kinase domain and Tyr929 in the SAM domain.
We have previously shown that the SH2 domain of Vav3 binds to EphA2 in a yeast two-hybrid system and in an in vitro binding assay (23). To determine the mechanism of interaction between EphA2 and Vav/p85 in mammalian cells, we performed co-immunoprecipitation assays using the SH2 domain of p85 or Vav3 or a Vav2ΔSH2 mutant. As shown in Fig. 4C, the SH2 domain of p85 or Vav3 was capable of binding to EphA2 receptor, whereas the Vav2ΔSH2 mutant failed to interact with EphA2, suggesting that interaction between EphA2 and Vav/p85 is mediated by binding between SH2 domains and Tyr(P) sites in EphA2. Taken together, our data suggest that Vav GEFs and p85 are major (but perhaps not the sole) binding partners of the EphA2 receptor.
Mutations That Uncouple EphA2 Receptor with Vav or p85 Inhibit Ephrin-A1-induced Rac1 GTPase Activation and Migration—Dynamic regulation of the actin cytoskeleton is critical in cell migration, and Rho family GTPases are known to be key regulators of this process and have been shown to be necessary for endothelial cell migration (31). We have previously reported that ephrin-A1 stimulation of endothelial cells induces activation of Rac1 GTPase through activation of guanine nucleotide exchange factors Vav2/Vav3 (23). In addition, ephrin-A1-induced Rac1 activation is dependent on the activity of PI 3-kinase (17). Since Tyr587/Tyr593 and Tyr734 are required for recruitment of Vav GEFs and p85, respectively (Fig. 4), we tested whether Y587E/Y593E and Y734F could affect ephrin-A1-induced Rac1 activation in endothelial cells. Cells were stimulated with ephrin-A1, and activated GTP-bound Rac1 or Cdc42 was isolated from lysates by precipitation with Pak1 p21-binding domain-glutathione S-transferase fusion proteins. As shown in Fig. 5A, consistent with our previous findings (17), ephrin-A1 induced Rac1 activation in EphA2-null endothelial cells reconstituted with wild-type EphA2 but not in control LZRS-infected cells. In contrast, Y587E/Y593E, Y734F, and Y929F mutants failed to restore Rac1-GTP level, suggesting that recruitment of p85 and Vav proteins to the EphA2 receptor is critical for ephrin-A1-induced Rac1 activation. Activated Cdc42 was not significantly changed in response to ephrin-A1 stimulation in either wild type or EphA2 mutants (Fig. 5B), indicating that EphA2 is not directly involved in regulating Cdc42 activity.
FIGURE 5.

Phosphorylation of Tyr587/Tyr593 and Tyr734 in the EphA2 receptor is required for EphA2-dependent Rac1 activation and cell migration. Active GTP-bound forms of Rac1 (A) and Cdc42 (B) were analyzed by Pak-1 binding domain pull-down followed by immunoblot in lysates from EphA2-null endothelial cells reconstituted with wild-type or mutant EphA2 in response to ephrin-A1 stimulation. Total Rac1 and Cdc42 levels within the lysates prior to Pak-1 binding domain pull-down were detected by immunoblot. Data are a representation of four independent experiments. C, EphA2-null endothelial cells reconstituted with Y587E/Y593E or Y734F mutant displayed significant reduced migration in response to ephrin-A1 stimulation in transwell migration assays (p < 0.01; EphA2 versus Y587E/Y593E or Y734F; two-tailed paired student t test). KO, knock-out.
Since activation of Rac1 is critical for ephrin-A1-induced endothelial cell migration (17), we tested whether cell migration is impaired in EphA2-null endothelial cells reconstituted with uncoupling EphA2 mutants. As shown in Fig. 5C, EphA2-null endothelial cells exhibit a defect in ephrin-A1-induced cell migration. Reexpression of wild-type EphA2 receptor rescued migration defects in EphA2 knock-out endothelial cells. In contrast, expression of Y587E/Y593E or Y734F mutants failed to promote ephrin-A1-induced cell migration, suggesting that phosphorylation of Tyr587/Tyr593 and Tyr734 is critical for recruitment of the p85 subunit of PI 3-kinase and Vav GEFs, which transduce downstream signaling to activate Rac1 GTPase and cell migration.
Functions of EphA2 Phosphorylated Tyrosine Residues in Vascular Assembly and Tumor Angiogenesis—Angiogenesis is a complex, multistage process by which new blood vessels are formed from preexisting vasculature. Two critical steps in this process are endothelial cell migration and assembly into new tubules. To test the functional roles of phosphorylated tyrosine residues of EphA2 receptor in ephrin-A1-induced angiogenic responses, we measured the vascular assembly in EphA2-null endothelial cells reconstituted with EphA2 mutants. Ephrin-A1 stimulation induced wild-type, but not EphA2-deficient, endothelial cell assembly into an interconnected vascular network on a thin layer of Matrigel. Reexpression of wild-type EphA2, but not empty control vector, by LZRS retrovirus-mediated infection rescued defects in EphA2-null endothelial cells. Likewise, expression of mutant Y593E or Y771F in EphA2-null cells restored the ability of cells to assemble and form interconnecting cellular network on Matrigel. In contrast, EphA2 mutations in the juxtamembrane (Y587F, Y587E/Y593E), kinase domain (Y734F), or SAM domain (Y929F) inhibited ephrin-A1-induced vascular assembly (Fig. 6).
FIGURE 6.

Phosphorylation of Tyr587/Tyr593, Tyr734, and Tyr929 in the EphA2 receptor is required for vascular assembly in vitro. Top, EphA2-null endothelial cells reconstituted with wild-type (WT) or mutant (knockout; KO) EphA2 were plated on a thin layer of growth factor reduced Matrigel in the presence of ephrin-A1 to examine and quantify vascular assembly. After 9 h, the endothelial cells were photographed. Bottom, average branch length was scored using morphometric software analysis. Four fields per culture were scored for each condition, and data are means ± S.D. of three independent experiments (p < 0.05; EphA2 versus Y587F, Y593F, Y587E/Y593E, Y734F, or Y929F; two-tailed paired Student's t test).
To test whether phosphorylated tyrosines important in mediating vascular assembly in vitro are also critical in tumor angiogenesis in vivo, we performed tumor cell/endothelial cell co-transplantation experiments using EphA2-null endothelial cells reconstituted with wild-type or mutant EphA2 receptors. For co-transplantation, endothelial cells were infected with adenoviruses encoding nuclear β-galactosidase (Ad-β-galactosidase) in order to distinguish them from endogenous host endothelium. These labeled endothelial cells were then co-transplanted with 4T1 mammary carcinoma cells in Matrigel into the subcutaneous dorsal flank of nude female mice. After 7 days, tumor-endothelial cell Matrigel plugs were harvested, sectioned, and double-stained with X-gal and CD31 to identify donor endothelial cells.
As shown in Fig. 7, significant numbers of LacZ-positive donor endothelial cells reconstituted with either wild type or Y921F control EphA2 mutant have incorporated into tumor (A) or peripheral vessels (B). In contrast, EphA2-deficient donor endothelial cells as well as cells reconstituted with Y587E/Y593E, Y734F, or Y929F, remained isolated and failed to incorporate into tumor vasculature. In addition, tumor volume was significantly increased in tumors harboring donor endothelial cells reconstituted with wild-type or Y921F control EphA2, relative to tumors containing EphA2-deficient endothelial cells or cells reconstituted with Y587E/Y593E, Y734F, or Y929F (Fig. 7C). Taken together, these data suggest that phosphorylated tyrosine residues, Tyr587/Tyr593, Tyr734, and Tyr929, are critical in EphA2 signal transduction and tumor angiogenesis in vivo.
FIGURE 7.
Phosphorylation of Tyr587/Tyr593, Tyr734, and Tyr929 in the EphA2 receptor is required for efficient incorporation of endothelial cells into tumor vasculature in vivo. 4T1 tumor cells were mixed with Ad-LacZ-transduced EphA2-null endothelial cells reconstituted with wild-type or mutant EphA2 in Matrigel and co-transplanted subcutaneously into BALB/c nude female mice. Tumors were collected 7 days post-transplantation. A and B, tumor sections were co-stained with X-gal (blue) and anti-CD31 antibodies (brown in B) to visualize donor endothelium and counterstained with eosin to visualize tumor cells (pink). Donor endothelial cells derived from EphA2-null reconstituted with wild-type EphA2 or control Y921F mutant coalesced around tumor cell clusters and displayed an elongated phenotype typical of endothelial cells. In contrast, endothelial cells derived from EphA2-null cells expressing Y734F mutant or control vector LZRS remained isolated and failed to incorporate into tumor vessels. The arrowheads indicate exogenous endothelial cells, and asterisks indicate the central lumens of chimeric vessels in B. C, tumor volume was significantly decreased for tumors harboring EphA2-null endothelial cells expressing Y587E/Y593E, Y734F, or Y929 mutant or control LZRS vector, relative to tumors co-transplanted with those expressing wild-type EphA2 or control Y921F mutant(p < 0.05; EphA2 versus Y587E/Y593E, Y734F, or Y929F; two-tailed paired student t test). Data are representative of three independent experiments. KO, knock-out.
DISCUSSION
A wealth of evidence has demonstrated that ephrin-A ligand stimulation of EphA2 receptors activates a signaling cascade that modulates actin cytoskeleton dynamics and regulates cell-cell and cell-matrix adhesion and cell motility (17, 23, 33). Phosphorylated tyrosine residues on the EphA2 receptor were thought to play a critical role in the recruitment of SH2 or PTB domain-containing signaling molecules, such as the p85 subunit of PI 3-kinase (24), adaptor proteins SLAP (34) and Shc (35), tyrosine phosphatase SHP-2 (36) and low molecular weight protein-tyrosine phosphatase (37, 38), ubiquitin ligase c-Cbl (39, 40), and guanine nucleotide exchange factors Vav2 and Vav3 (23). In the endothelial cells, we have previously shown that either PI 3-kinase inhibitors or a dominant negative p85 mutant significantly inhibited ephrin-A1 ligand-induced endothelial cell migration (17). Likewise, Vav2/3-deficient endothelial cells were incapable of mediating cell migration and assembly upon ephrin-A1 stimulation (23). These data indicate important roles of PI 3-kinase and Vav GEFs in ephrin-A-elicited angiogenic responses. However, since these signaling molecules also act downstream of many receptor tyrosine kinases, it remains unclear whether recruitment of these proteins by EphA2 receptor is critical for endothelial cell function. As a first step to dissect the specific function of different phosphorylated tyrosine residues in the activated EphA2 receptor, we undertook mapping major tyrosine phosphorylation sites on the EphA2 receptor. We found that four of the 15 tyrosines (Tyr587, Tyr593, Tyr734, and Tyr771) in the EphA2 cytoplasmic domain were phosphorylated in vascular endothelial cells.
Two tyrosine residues in the juxtamembrane region of the EphB receptor were previously shown to be phosphorylated in vivo and are important in regulating kinase activity (21, 41). Of the corresponding two juxtamembrane tyrosine residues in the EphA2 receptor, we found that both Tyr587 and Tyr593 were phosphorylated in our in vitro kinase assay. However, only the phosphorylation of Tyr593 was detected in vivo in COS7 cells by LC-MS mass spectrometric analysis. This apparent discrepancy is most likely due to dephosphorylation of Tyr587 in vivo upon ephrin-A1 stimulation for 15 min. As predicted, Tyr587 plays a critical role in endothelial cell assembly in vitro and in endothelial cell incorporation into tumor vasculature in vivo (Figs. 6 and 7). Tyr593 was phosphorylated in COS7 cells and in vascular endothelial cells (Figs. 1, 2 and 3). Consistent with data shown in EphB receptor (21, 41), the Y593F mutation abolished kinase activity. Although the Y593E mutation inhibited binding of EphA2 to Vav GEFs (Fig. 4), it is somewhat surprising that it did not affect ephrin-A1-induced vascular assembly (Fig. 6). It is conceivable that residual levels of EphA2 binding to Vav GEFs through Tyr587 in vascular endothelial cells may be sufficient to transduce signals and regulate angiogenic responses.
For tyrosine residues in the kinase domain, Tyr771 resides in the activation loop of the kinase and was phosphorylated in both the in vitro kinase assay and in vivo as shown by mass spectrometric analysis (Figs. 1, 2 and 3). Interestingly, the Y771F mutant retains kinase activity, and no obvious phenotype was detected in our assays. Mass spectrometric analysis and two-dimensional phosphopeptide mapping also revealed a novel phosphorylated tyrosine residue, Tyr734. The significance of Tyr734 phosphorylation appears to recruit the p85 subunit of PI3 kinase (Fig. 4), since the Y734F mutant failed to rescue cell migration and vascular assembly in EphA2-deficient endothelial cells.
Of the three tyrosines in the SAM domain, Tyr921, Tyr929, and Tyr959, none of them was identified to be phosphorylated by either mass spectrometry or phosphopeptide mapping analysis. However, Y929F inhibited ephrin-A1-induced vascular assembly and endothelial cell incorporation into tumor vasculature in vivo (Figs. 6 and 7). Since Y929F also displays reduced kinase activity, the phenotype could be attributed to either lack of phosphorylation at the Tyr929 site or insufficient kinase activity, or a combination of both deficiencies. It is also possible that Tyr929 was not phosphorylated within 15 min of stimulation by ephrin-A1 but was phosphorylated in a different time frame. Alternatively, Tyr929 may not be phosphorylated in COS7 cells but may be phosphorylated in endothelial cells in vivo by protein-tyrosine kinases other than EphA2. Our binding data suggest that Vav3 and p85 interact with pY929 (Fig. 4). In addition, Stein et al. (42, 43) reported that low molecular weight protein-tyrosine phosphatase and Grb10 can bind to a corresponding site in the EphB1 receptor. If low molecular weight protein-tyrosine phosphatase also interacts with Tyr(P)929 in EphA2 receptor, it may attenuate its signaling by dephosphorylation of EphA2 receptor, as demonstrated in tumor cells (37, 38). Alternatively, low molecular weight protein-tyrosine phosphatase may transduce EphA2 signaling by interacting with p190RhoGAP to regulate the activity of Rho GTPases (45). It remains to be determined whether low molecular weight protein-tyrosine phosphatase can interact with EphA2 and modulate ephrin-A1-induced angiogenic responses.
Our results revealed that phosphorylated tyrosine residues in the EphA2 receptor are not only critical for signal transduction in cultured microvascular endothelial cells in vitro but also important for these cells to participate in tumor angiogenesis in vivo. It is interesting to note that tumors grow better in the presence of wild-type donor endothelial cells than EphA2-null cells or null cells reconstituted with Y587E/Y593E, Y734F, or Y929F mutants. This may not be entirely due to extra oxygen and nutrients supplied by new blood vessels, since tumor blood flow appeared to be restricted to peripheral vessels distant from the tumor mass. It is possible that there could be paracrine signaling from donor endothelial cells to tumor cells to promote tumor growth, and this signal(s) is absent or diminished in EphA2-null endothelial cells. Indeed, we have previously shown that ephrin-A1 regulates soluble growth factor production in tumor cells (44). The growth factors/signaling molecules that are modulated by EphA2 receptor activation in vascular endothelial cells remain to be determined.
In summary, mapping of phosphorylated tyrosine residues in the EphA2 receptor allowed us to generate tyrosine to phenylalanine mutants that were used to identify binding sites to key EphA2 downstream signaling molecules, such as the p85 subunit of PI3 kinase and Vav guanine nucleotide exchange factors. These uncoupling mutants permit the possibility to test whether recruitment of these proteins by EphA2 receptor is critical for endothelial cell function in vivo. As shown in Figs. 4 and 5, Tyr587/Tyr593 and Tyr734 are major sites for recruitment of Vav2/3 GEFs and p85, respectively, and the recruitment of both Vav proteins and p85 to the activated EphA2 receptor is critical for ephrin-A1-induced endothelial cell migration and assembly. It should be now feasible to screen for other putative EphA2 phosphotyrosine-dependent interacting proteins, such as Shc, SHP2, c-Cbl, and SLAP, or to use phosphopeptides as ligands in chromatography analysis to identify novel binding partners. Moreover, EphA2 tyrosine phosphospecific monoclonal antibodies can be developed to facilitate the identification of downstream signaling events associated with EphA2 tyrosine phosphorylation.
This work was supported, in whole or in part, by National Institutes of Health Grants CA95004 and CA114301 (to J. C.) and CA1179151 to (D. M. B.-S.). This work was also supported by Department of Defense Predoctoral Fellowship W81XWH-05-1-0254 (to W. B. F.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GEF, guanine nucleotide exchange factor; PI, phosphatidylinositol; LC, liquid chromatography; MS, mass spectrometry; SH2, Src homology 2; X-gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; TAg, T-antigen.
References
- 1.Brantley-Sieders, D., Schmidt, S., Parker, M., and Chen, J. (2004) Curr. Pharm. Des. 10 3431-3442 [DOI] [PubMed] [Google Scholar]
- 2.Pasquale, E. B. (2005) Nat. Rev. Mol. Cell. Biol. 6 462-475 [DOI] [PubMed] [Google Scholar]
- 3.Gale, N. W., Holland, S. J., Valenzuela, D. M., Flenniken, A., Pan, L., Ryan, T. E., Henkemeyer, M., Strebhardt, K., Hirai, H., and Wilkinson, D. G. (1996) Neuron 17 9-19 [DOI] [PubMed] [Google Scholar]
- 4.Adams, R. (2002) Semin. Cell Dev. Biol. 13 55-60 [DOI] [PubMed] [Google Scholar]
- 5.Brantley-Sieders, D., and Chen, J. (2004) Angiogenesis 7 17-28 [DOI] [PubMed] [Google Scholar]
- 6.Gerety, S. S., and Anderson, D. J. (2002) Development 129 1397-1410 [DOI] [PubMed] [Google Scholar]
- 7.Foo, S. S., Turner, C. J., Adams, S., Compagni, A., Aubyn, D., Kogata, N., Lindblom, P., Shani, M., Zicha, D., and Adams, R. H. (2006) Cell 124 161-173 [DOI] [PubMed] [Google Scholar]
- 8.Noren, N. K., Lu, M., Freeman, A. L., Koolpe, M., and Pasquale, E. B. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 5583-5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erber, R., Eichelsbacher, U., Powajbo, V., Korn, T., Djonov, V., Lin, J., Hammes, H. P., Grobholz, R., Ullrich, A., and Vajkoczy, P. (2006) EMBO J. 25 628-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel, T. O., Stein, E., Cerretti, D. P., John, P. L., Robert, B., and Abrahamson, D. R. (1996) Kidney Int. Suppl. 57 73-81 [PubMed] [Google Scholar]
- 11.Ogawa, K., Pasqualini, R., Lindberg, R. A., Kain, R., Freeman, A. L., and Pasquale, E. B. (2000) Oncogene 19 6043-6052 [DOI] [PubMed] [Google Scholar]
- 12.Pandey, A., Shao, H., Marks, R. M., Polverini, P. J., and Dixit, V. M. (1995) Science 268 567-569 [DOI] [PubMed] [Google Scholar]
- 13.Cheng, N., Brantley, D. M., Liu, H., Lin, Q., Enriquez, M., Gale, N. W., Yancopoulos, G., Cerretti, D. P., Daniel, T. O., and Chen, J. (2002) Mol. Cancer Res. 1 2-11 [PubMed] [Google Scholar]
- 14.Brantley, D. M., Cheng, N., Thompson, E. J., Lin, Q., Brekken, R. A., Thorpe, P. E., Muraoka, R. S., Cerretti, D. P., Pozzi, A., Jackson, D., Lin, C., and Chen, J. (2002) Oncogene 21 7011-7026 [DOI] [PubMed] [Google Scholar]
- 15.Cheng, N., Brantley, D., Liu, H., Fanslow, W., Cerretti, D. P., Reith, A. D., Jackson, D., and Chen, J. (2003) Neoplasia 5 445-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrzanski, P., Hunter, K., Jones-Bonlin, S., Chang, H., Robinson, C., Pritchard, S., Zhao, H., and Ruggeri, B. (2004) Cancer Res. 64 910-919 [DOI] [PubMed] [Google Scholar]
- 17.Brantley-Sieders, D., Caughron, J., Hicks, D., Pozzi, A., Ruiz, J. C., and Chen, J. (2004) J. Cell Sci. 117 2037-2049 [DOI] [PubMed] [Google Scholar]
- 18.Brantley-Sieders, D. M., Fang, W. B., Hicks, D., Koyama, T., Shyr, Y., and Chen, J. (2005) FASEB J. 19 1884-1886 [DOI] [PubMed] [Google Scholar]
- 19.Kalo, M. S., and Pasquale, E. B. (1999) Biochemistry 38 14396-14408 [DOI] [PubMed] [Google Scholar]
- 20.Kalo, M. S., Yu, H. H., and Pasquale, E. B. (2001) J. Biol. Chem. 276 38940-38948 [DOI] [PubMed] [Google Scholar]
- 21.Binns, K. L., Taylor, P. P., Sicheri, F., Pawson, T., and Holland, S. J. (2000) Mol. Cell. Biol. 20 4791-4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrenson, I. D., Wimmer-Kleikamp, S. H., Lock, P., Schoenwaelder, S. M., Down, M., Boyd, A. W., Alewood, P. F., and Lackmann, M. (2002) J. Cell Sci. 115 1059-1072 [DOI] [PubMed] [Google Scholar]
- 23.Hunter, S. G., Zhuang, G., Brantley-Sieders, D. M., Swatt, W., Cowan, C. W., and Chen, J. (2006) Mol. Cell. Biol. 26 4830-4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey, A., Lazar, D. F., Saltiel, A. R., and Dixit, V. M. (1994) J. Biol. Chem. 269 30154-30157 [PubMed] [Google Scholar]
- 25.Ham, A.-J. (2005) in The Encyclopedia of Mass Spectrometry: Biological Proteins and Peptides (Gross, M. L., and Caprioli, R. M., eds) pp. 10-17, Elsevier Ltd., Kidlington, UK
- 26.Eng, J. K., McCormack, A. L., and Yates, J. R. I. (1994) J. Am. Soc. Mass Spectrom. 5 976-989 [DOI] [PubMed] [Google Scholar]
- 27.Yates, J. R., Eng, J. K., McCormack, A. L., and Schieltz, D. (1995) Anal. Chem. 67 1426-1436 [DOI] [PubMed] [Google Scholar]
- 28.Hansen, B. T., Davey, S. W., Ham, A.-J. L., and Liebler, D. C. (2005) J. Proteome Res. 4 358-368 [DOI] [PubMed] [Google Scholar]
- 29.Jat, P. S., Noble, M. D., Ataliotis, P., Tanaka, Y., Yannoutsos, N., Larsen, L., and Kioussis, D. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 5096-5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang, W. B., Brantley-Sieders, D. M., Parker, M. A., Reith, A. D., and Chen, J. (2005) Oncogene 24 7859-7866 [DOI] [PubMed] [Google Scholar]
- 31.Ridley, A. J. (2001) J. Cell Sci. 114 2713-2722 [DOI] [PubMed] [Google Scholar]
- 32.Nor, J. E., Peters, M. C., Christensen, J. B., Sutorik, M. M., Linn, S., Khan, M. K., Addison, C. L., Mooney, D. J., and Polverini, P. J. (2001) Lab. Invest. 81 453-463 [DOI] [PubMed] [Google Scholar]
- 33.Miao, H., Nickel, C. H., Cantley, L. G., Bruggeman, L. A., Bennardo, L. N., and Wang, B. (2003) J. Cell Biol. 162 1281-1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey, A., Duan, H., and Dixit, V. M. (1995) J. Biol. Chem. 270 19201-19204 [DOI] [PubMed] [Google Scholar]
- 35.Pratt, R. L., and Kinch, M. S. (2002) Oncogene 21 7690-7699 [DOI] [PubMed] [Google Scholar]
- 36.Miao, H., Burnett, E., Kinch, M., Simon, E., and Wang, B. (2000) Nat. Cell Biol. 2 62-69 [DOI] [PubMed] [Google Scholar]
- 37.Kikawa, K., Vidale, D. R., Van Etten, R. L., and Kinch, M. S. (2002) J. Biol. Chem. 277 39274-39279 [DOI] [PubMed] [Google Scholar]
- 38.Chiarugi, P., Cirri, P., Marra, F., Raugei, G., Camici, G., Manao, G., and Ramponi, G. (1997) Biochem. Biophys. Res. Commun. 238 676-682 [DOI] [PubMed] [Google Scholar]
- 39.Walker-Daniels, J., Riese, D. J., and Kinch, M. S. (2002) Mol. Cancer Res. 1 79-87 [PubMed] [Google Scholar]
- 40.Wang, Y., Ota, S., Kataoka, H., Kanamori, M., Li, Z., Band, H., Tanaka, M., and Sugimura, H. (2002) Biochem. Biophys. Res. Commun. 296 214-220 [DOI] [PubMed] [Google Scholar]
- 41.Wybenga-Groot, L. E., Baskin, B., Ong, S. H., Tong, J., Pawson, T., and Sicheri, F. (2001) Cell 106 745-757 [DOI] [PubMed] [Google Scholar]
- 42.Stein, E., Cerretti, D. P., and Daniel, T. O. (1996) J. Biol. Chem. 271 23588-23593 [DOI] [PubMed] [Google Scholar]
- 43.Stein, E., Lane, A. A., Cerretti, D. P., Schoeklmann, Schroff, A. D., Van Etten, R. L., and Daniel, T. O. (1998) Genes Dev. 12 667-678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brantley-Sieders, D., Fang, W. B., Hwang, Y., Hicks, D., and Chen, J. (2006) Cancer Res. 66 10315-10324 [DOI] [PubMed] [Google Scholar]
- 45.Fang, W. B., Ireton, R. C., Zhuang, G., Takahashi, T., Reynolds, A. B., and Chen, J. (2008) J. Cell Sci. 121 358-368 [DOI] [PubMed] [Google Scholar]