Abstract
Cell migration in blood flow is mediated by engagement of specialized adhesion molecules that function under hemodynamic shear conditions, and many of the effectors of these adhesive interactions, such as the selectins and their ligands, are well defined. However, in contrast, our knowledge of the adhesion molecules operant under lymphatic flow conditions is incomplete. Among human malignancies, head and neck squamous cell cancer displays a marked predilection for locoregional lymph node metastasis. Based on this distinct tropism, we hypothesized that these cells express adhesion molecules that promote their binding to lymphoid tissue under lymphatic fluid shear stress. Accordingly, we investigated adhesive interactions between these and other cancer cells and the principal resident cells of lymphoid organs, lymphocytes. Parallel plate flow chamber studies under defined shear conditions, together with biochemical analyses, showed that human head and neck squamous cell cancer cells express heretofore unrecognized L-selectin ligand(s) that mediate binding to lymphocyte L-selectin at conspicuously low shear stress levels of 0.07–0.08 dynes/cm2, consistent with lymphatic flow. The binding of head and neck squamous cancer cells to L-selectin displays canonical biochemical features, such as requirements for sialylation, sulfation, and N-glycosylation, but displays a novel operational shear threshold differing from all other L-selectin ligands, including those expressed on colon cancer and leukemic cells (e.g. HCELL). These data define a novel class of L-selectin ligands and expand the scope of function for L-selectin within circulatory systems to now include a novel activity within shear stresses characteristic of lymphatic flow.
All cell-cell and cell-matrix interactions in nature require engagement of adhesion molecules capable of resisting prevailing forces of shear, either present within the organism or in the microenvironmental milieu. Cell lodgment and tissue residence thus require the presence of adhesion molecules capable of mediating binding within the shear stresses inherent to a particular site. Within organisms possessing circulatory systems, the shear force to be resisted in order to establish biologically relevant interactions is smallest within interstitial compartments and greatest within the hemovascular compartment. At present, our knowledge regarding the molecular basis of leukocyte hematogenous trafficking to lymph node, skin, and acute inflammatory sites is relatively robust (1, 2). Most commonly, leukocytes exit the vasculature at post-capillary venules, where shear stress ranges from 1 to 4 dynes/cm2 (1, 2). The well established “multistep” paradigm of leukocyte trafficking holds that leukocytes in flow must first make contact along the endothelial surface with adhesive interactions of sufficient strength to overcome the shear forces of blood flow. The principal effectors of the initial leukocyte adhesion to endothelium is the selectin family of adhesion molecules. The selectins (E-, P-, and L-selectins, CD62E, CD62P, and CD62L, respectively) are a family of calcium-dependent glycoproteins that are the most efficient mediators of shear-resistant interactions described to date. Each of the selectins displays optimal binding to its respective ligands under physiologic shear conditions, particularly once a shear threshold has been surpassed (3). E- and P-selectin are expressed on the vascular endothelium, and P-selectin is also expressed on platelets (4). L-selectin, however, is strictly expressed on leukocytes and is highly expressed on peripheral blood lymphocytes, particularly among naive and central memory lymphocytes (4, 5). Initial selectin-mediated interaction enables subsequent engagement of chemokine receptors and integrins that promote cellular firm adhesion, endothelial transmigration, and ultimately target site residence. It is known that similar receptor/ligand cascades, also initiated by selectin-mediated interactions, promote homing of hematopoietic stem cells to bone marrow as well as tumor cell hematogenous targeting of distant metastatic sites (2, 6–8). In contrast, however, the process of cellular trafficking within lower fluid shear stress levels such as in lymphatic compartments is poorly understood.
Tumor spread to lymph nodes is the culmination of a multistep process that includes tumor cell invasion into the lymphovascular compartment, tumor cell lodgment within the targeted tissue, and tumor cell growth within this new microenvironment. Recent reports have shed light on the initial steps driving the process of lymphatic metastasis (9–12) and of physiologic cellular recruitment into lymphatic vessels (13). However, the molecular basis of tumor cell lodgment within lymph nodes is uncharacterized. In head and neck squamous cell cancer (HNSCC),4 as opposed to most other solid tumors, spread of disease is overwhelmingly confined to regional lymph nodes, with distant metastatic disease developing only as a late feature of the most advanced cases (14). This distinctive clinical characteristic prompted us to analyze whether HNSCC cells possess unique molecular effectors that mediate adhesive interactions active within lymphovascular shear stress, such as those that may be important for tumor cell lodgment within the lymph node microenvironment. These cellular interactions occur in the setting of lymph flow and in the absence of an endothelial barrier. Thus, cells entering the lymph node via afferent lymphatic flow percolate within the nodal parenchyma, a compartment composed primarily of lymphocytes.
Measurements of shear stresses within the lymph node microenvironment have not been reported. However, measurements of flow within human lymphatic capillaries in vivo have recorded lymph median linear velocity to be 0.097 mm/s (15). Based on this value, and the similar dimensions of measured lymphatic capillaries and lymph node sinuses, mean wall physiological shear stress within the lymph node sinuses is estimated to be 0.08 dynes/cm2, 10-fold lower than hematogenous shear stress levels. Within this backdrop of motion, we hypothesized that cells entering the lymph node interstitial microenvironment that express adhesion receptors specialized to function within this shear stress would have an advantage in establishing residence. Thus, we examined whether HNSCC cells are specialized to bind lymphocytes under low fluid shear. Here we show that HNSCC cells interact with L-selectin expressed on lymphocytes under conditions of shear stress, but not under static conditions. This interaction is maximal within low shear stress levels characteristic of lymph flow and is mediated by a novel class of L-selectin ligands expressed on primary HNSCC cells. This adhesive phenotype is characteristic of HNSCC but is not displayed by other solid tumor cells. Our findings unveil a previously unrecognized role for L-selectin in mediating lymphocyte-adhesive interactions under low shear stress (<1 dyne/cm2) and provide new perspectives on shear stress-based biology as may occur within the lymphatic system.
EXPERIMENTAL PROCEDURES
Cells and Reagents—Head and neck cancer cell lines JHU-SCC-011, JHU-SCC-013, and JHU-SCC-019 were a gift from Dr. James Rocco (Boston). These cell lines were developed from tumors in patients diagnosed with squamous cell carcinoma of the upper aerodigestive tract (16, 17). KG1a, LS174T, MCF-7, and MDA-MB-231 cells were purchased from ATCC (Manassas, VA). Cells were maintained in RPMI 1640 medium containing glutamine supplemented with 10% fetal bovine serum (FBS) under standard cell culture conditions. Human lymphocytes were purified from blood by isolation of the mononuclear cell fraction from peripheral blood (PBMCs). PBMCs were prepared by density gradient separation (Histopaque 1077, Sigma) of peripheral blood obtained from healthy donors with full consent as per an Institutional Human Subjects Internal Review Board-approved protocol. Isolated PBMC were >90% T cells (CD3+ cells) and expressed L-selectin as determined by immunofluorescent staining and flow cytometry using a Beckman-Coulter model Cytomics FC 500 MPL (Fullerton, CA). Enzymes and chemical reagents used are as follows: α2–3-neuraminidase from Streptococcus pneumoniae (Calbiochem), α2–3,6,8-neuraminidase from Vibrio cholerae (Roche Diagnostics), O-sialoglycoprotein endopeptidase (Cedarlane, Ontario, Canada), heparinase II (Sigma), sodium chlorate (Sigma), tunicamycin (Sigma), phorbol 12-myristate 13-acetate (Sigma), and bromelain (Sigma). Recombinant human L-selectin/human Ig Fc chimera was purchased from R & D Systems. Antibodies used in the study were as follows: rat anti-human CLA, HECA-452, IgM (Pharmingen); MECA-79, rat IgM (kind gift from Dr. Phillip R. Streeter, Oregon Health Sciences University); murine anti-human PSGL-1, KPL-1, IgG1 (Pharmingen); mouse IgG1,κ isotype control (Pharmingen); rat IgM isotype control (Pharmingen); murine anti-human CD44, 2C5, IgG2a (R & D Systems, Minneapolis, MN); anti-L-selectin antibody LAM1-116, mIgG2a (Research Diagnostics Inc., Concord, MA); murine anti-human cytokeratin antibody, MAB3412, IgG1 (Chemicon, Temecula, CA); Texas red-conjugated goat anti-mouse IgG antibody (Pierce); fluorescein isothiocyanate-conjugated goat anti-mouse Ig and fluorescein isothiocyanate-conjugated goat anti-rat IgM (Pharmingen);. rat anti-human CD44, Hermes-1, IgG2a was a gift of Dr. Brenda Sandmaier (Fred Hutchinson Cancer Research Center; Seattle, WA). Purified and phycoerythrin-conjugated murine anti-human CD34, QBEND10, IgG1, and phycoerythrin-conjugated mouse IgG1,κ isotype control were from Coulter-Immunotech (Miami, FL). Alkaline phosphatase-conjugated anti-rat IgM and anti-mouse Ig were from Southern Biotechnology Associates, Birmingham, AL.
Shear-dependent Binding Assays—Cell-cell interactions were studied by growing cells in standard tissue culture-treated 6-well plates. A circular parallel plate flow chamber apparatus (GlycoTech, Gaithersburg, MD) with internal flow chamber dimensions of 2 × 0.5 × 0.025 cm was mounted over the unfixed live cell monolayer (HNSCC cells) or fixed cell monolayer (KG1a and LS174T cells) within a well and sealed using low wall suction as described previously (18, 19). The system was then equilibrated with Hanks' balanced salt solution (Invitrogen) supplemented with 10 mm HEPES, pH 7.4, and 2 mm CaCl2 (binding buffer). Purified lymphocytes were washed and resuspended in binding buffer at a concentration of 4.0 × 106 cells/ml. Unfixed lymphocytes were drawn into the chamber under defined flow conditions by a precision syringe pump (Harvard Apparatus, Cambridge, MA), and interactions were observed in real time under stable shear force conditions using an inverted phase contrast microscope. For experiments titrating shear stresses, the lymphocytes were first introduced into the chamber at 0.5 ml/min and then flow was momentarily suspended allowing for cells to settle, followed by an incremental increase in flow rate. Runs were recorded onto videotape using a standard CCD camera attached to a VHS recorder. Interactions were scored by review of the videotape. Primary attachments were quantified by determining the number of lymphocytes that interact with the cancer cell monolayer for more than two video frames (0.07 s) during the first 3 min of flow. Secondary interactions from cells that rolled into the field of observation from upstream regions were excluded. Rolling was defined as lateral translation of a bound cell greater than five cell diameters below the hydrodynamic velocity. Firm adhesion was defined as no lateral translation in the setting of hydrodynamic velocity and interaction resistant to shear force. Accumulation was defined as the sum of rolling and firm adhesion interactions and was scored after 4 min of flow. L-selectin specificity of interactions were established by pretreating lymphocytes with anti-L-selectin antibody LAM1-116 or anti-L-selectin polyclonal serum (50 μg/ml, on ice, 30 min) or PMA (10 ng/ml, 37 °C, 1 h).
Alternatively, unfixed cancer cells in suspension were also introduced in the flow chamber over L-selectin chimera adsorbed to the tissue culture plate. Cells were grown under standard culture conditions and harvested by incubation with EDTA (5 mm EDTA, 37 °C, 15 min) and washed with binding buffer. Cells were resuspended to a final concentration of 1.0 × 106 cells/ml. Chimera plates were prepared by overnight incubation with L-selectin chimera (0.5 μg/ml in PBS) at 4 °C and blocked with heat-inactivated FBS for 24 h. Attachment was assayed by introducing tumor cells into the parallel flow chamber at 0.5 ml/min (1.4 dynes/cm2) followed by decreasing flow to the desired level. In experiments shown in Fig. 2A, flow was incrementally decreased every 30 s. In other experiments, flow was immediately decreased to the desired level once the cell bolus was visualized in the field. Accumulation was scored at the end of 30 s of flow at a determined shear stress. The specificity of L-selectin interaction was assessed by pretreating the cancer cells with bromelain (0.1%, 37 °C, 1 h), EDTA (10 mm EDTA, 30 min), neuraminidase (0.1 unit/ml at 37 °C, 1 h), O-sialoglycoprotein endopeptidase (24 or 240 μg/ml at 37 °C, 2 h), heparinase (0.05 or 5 units/ml, 37 °C, 4 h), sodium chlorate (10 mm, 37 °C, 20 h), or tunicamycin (15 μg/ml, 37 °C, 20 h.). L-selectin specificity of interactions was established by pretreating the prepared L-selectin chimera plates with anti-L-selectin antibody LAM1-116 or anti-L-selectin polyclonal serum (50 μg/ml) prior to the infusion of the cancer cell suspensions. Mouse isotype antibody was used as negative controls for blocking. Detachment was assayed by first establishing binding at 0.07 dynes/cm2 and then incrementally raising flow rate. Adhesive interactions were scored 30 s after establishing a discrete shear stress level. Rolling velocity was calculated as the distance traveled by the centroid of an attached cell divided by the period of observation (5 s).
FIGURE 2.
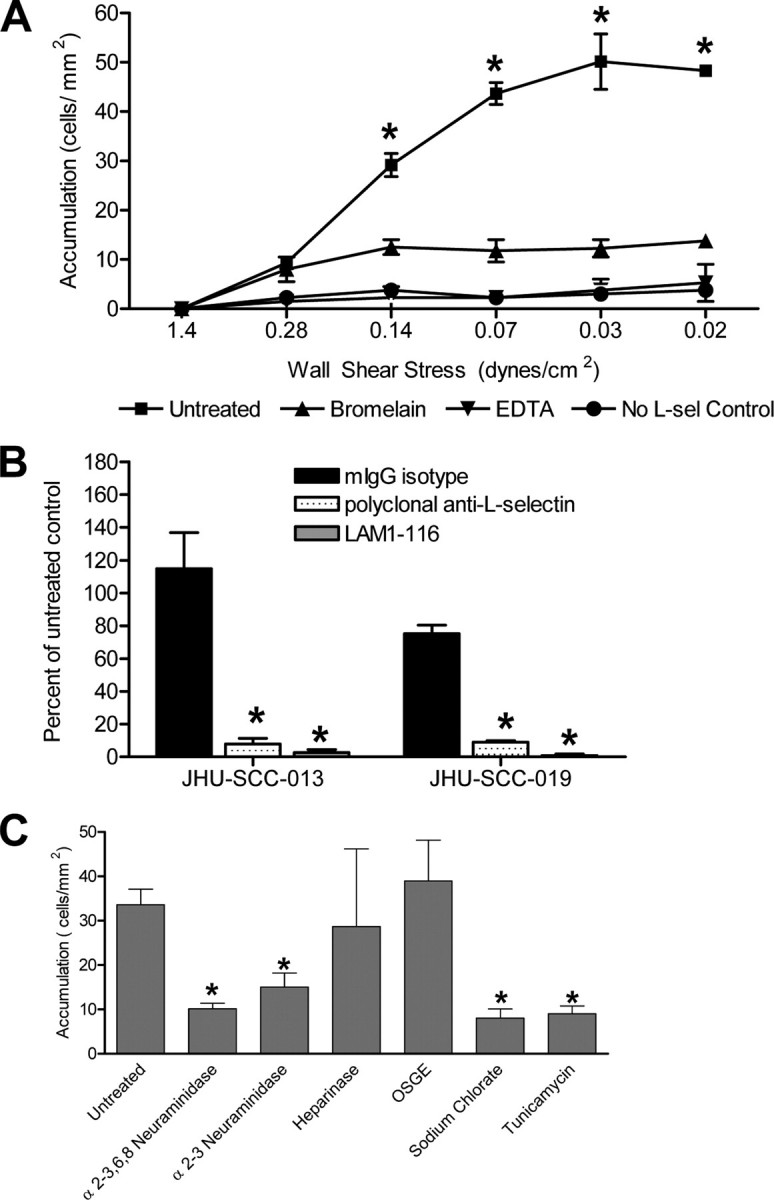
HNSCC cells in flow express novel L-selectin ligands. A, JHU-SCC-019 cells were introduced in flow at 0.5 ml/min into the parallel plate flow chamber containing adhered L-selectin/Fc chimera. Flow was then decreased incrementally to the desired levels as indicated in the figure. Cells in flow interact with adsorbed L-selectin (L-sel) chimera at low shear stress. The interaction is sensitive to bromelain treatment of cells and to chelation of divalent cations with EDTA. No chimera control confirms specificity of the assay. * represents statistically significant difference from EDTA-treated, bromelain-treated, and no chimera controls. B, interaction between JHU-SCC-019 cells and the L-selectin/Fc chimera is blocked by pretreating the solid surface containing the adherent chimera with blocking anti-L-selectin antibodies. * represents statistically significant difference from isotype control. C, JHU-SCC-019 cell binding to L-selectin chimera is sensitive to treatment with broad neuraminidase (α2–3,6,8) and α2–3-specific neuraminidase, as well as to treatment with sodium chlorate and tunicamycin suggesting the presence of important sialylated, sulfated, and N-linked glycosylated determinants. Error bars represent the mean ± S.E. * represents statistically significant difference from untreated. Differences between sialidase treatments, sodium chlorate treatment, and tunicamycin treatment are not statistically significant. OSGE, O-sialoglycoprotein endopeptidase.
All experiments were performed on different days with the use of freshly prepared reagents, i.e. newly isolated lymphocytes, newly isolated cancer cell lines, or newly created L-selectin chimera spots. Wall shear stress (T) values were calculated according to the formula T (dynes/cm2) = 3 μQ/2ba2, where μ is the coefficient of viscosity of the solution in the chamber (poise); Q is the volumetric flow rate (cm3/s); b is the channel width (0.5 cm); and a is the half-channel height (0.0127 cm). A value of 0.009 poise (for water) was used for the viscosity of flow buffer at 25 °C.
Primary Tumor-based Assays—Fresh primary tumor specimens were dissected from the center of the tumor mass (i.e. not bordering on normal mucosa) and were minced and incubated in RPMI containing 60 μg/ml collagenase (Sigma C9697) at 37 °C for 3–6 h. Undigested tumor pieces were allowed to briefly settle to the bottom of the tube, and cells in suspension were removed with the supernatant. Cells were then pelleted at 200 × g for 10 min and resuspended in 3–6 ml of Hanks' balanced salt solution (Invitrogen) supplemented with 10 mm HEPES, pH 7.4, and 2 mm CaCl2 (binding buffer). Cells were examined for low shear-dependent L-selectin binding as described above using the adherent L-selectin chimera assay. Adhered human immunoglobulin (Miles Laboratories, West Haven, CT) (0.5 μg/ml in PBS) or FBS was used as negative control for binding. Cells were introduced into the parallel plate chamber at 0.07 dynes/cm2 for 5 min, followed by a 1-ml wash with binding buffer introduced at 0.3 dynes/cm2, and fixation with 1 ml of 4% phosphate-buffered paraformaldehyde introduced at 0.3 dynes/cm2. Fixed cells were stored at 4 °C until stained. Plates containing previously fixed cells were treated with PBS, 0.5% Triton X-100 for 5 min, followed by a PBS rinse. Plates were blocked for 15 min in 1× PBS, 5% normal horse serum, 1% bovine serum albumin and then incubated with anti-cytokeratin antibody for 30 min. Following PBS rinses, the plates were incubated with a 1:200 dilution of Texas red-conjugated goat anti-mouse antibody for 30 min at 25 °C. Following a PBS rinse, the plates were incubated with 0.4 μg/ml 4′,6-diamidino-2-phenylindole (Pierce) in PBS for 5 min and rinsed, and coverslips were mounted with Vectashield (Vector Laboratories, Burlingame, CA). Cells were examined, and digital pictures were taken using a Nikon TE2000-U microscope with Photometrics Cool SNAP EZ digital camera (Roper Scientific, Tucson, AZ) and the NIS Elements BR software (Nikon, Melville, NY).
Selectin Ligand Expression—The expression of CD34, PSGL-1, HECA-452, and MECA-79 was assessed by standard flow cytometry. Expression of HCELL was assessed by immunoprecipitating CD44 with the Hermes-1 antibody followed by Western blot evaluation of the immunoprecipitate with the HECA-452 antibody as described previously (19). Briefly, membrane protein lysates of the KG1a and HNSCC cell lines were prepared in 2% Nonidet P-40, Buffer A (1% SDS, 150 mm NaCl, 0.5 mm Tris, pH 10.4, 1 mm EDTA, 20 μg/ml phenylmethylsulfonyl fluoride) and incubated with immunoprecipitating antibodies or with appropriate isotype controls overnight at 4 °C. The antibody/lysate mixture was then immunoprecipitated by the addition of protein G-agarose beads (Invitrogen) followed by extensive washes with immunoprecipitating buffer. The pellet was then treated with 6× reducing gel loading buffer, boiled, and the supernatant recovered. The samples were then resolved by standard SDS-PAGE using 7.5% Tris-HCl pre-cast gels (Bio-Rad). The gels were subsequently transferred onto polyvinylidene difluoride blot membrane (Millipore, Billerica, MA) and blocked using standard Western blot technique. The blots were treated with primary antibody (1 μg/ml) and alkaline phosphatase-linked secondary antibody (1:1000). Bands were visualized using the Western blue alkaline phosphatase substrate system (Promega, Madison, WI).
Statistics—Statistical analysis was performed using single factor analysis of variance, as well as Tukey post hoc pairwise test. Statistical significance was accepted for p values less than 0.05. All p values were two-tailed. A minimum of three independent observations were made for each measurement.
RESULTS
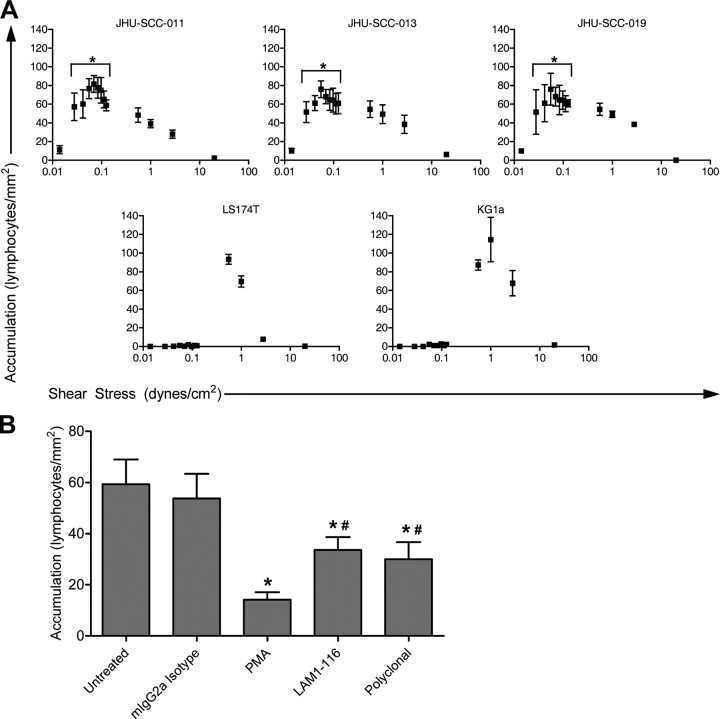
Lymphocytes Bind to HNSCC Cells under Conditions of Low Fluid Shear—To analyze whether HNSCC cells interact with lymphocytes under shear stress, parallel plate flow chamber binding assays were used. This experimental system has been extensively used for the study of shear stress-dependent adhesion molecule biology (7, 20–24). Human PBMCs from normal donors were used as a source of lymphocytes (>90% lymphocytes). Head and neck cancer cells were grown on a solid support, and the lymphocytes were delivered under flow at discrete shear stresses by use of a standard syringe pump to define the latter. This experimental design avoids membrane fixation and thus measures the interaction(s) of the physiologically presented cell surface adhesion molecules, in native conformations, within the lipid bilayer. Adhesive interaction events were captured on videotape by using a standard CCD camera and video recording assembly. Events were scored on video as rolling events or firm adhesion events (see “Experimental Procedures”). Lymphocytes were introduced into the flow chamber at 0.6 dynes/cm2. The flow velocity was incrementally increased every 30 s up to 20 dynes/cm2. Under these conditions, HNSCC cell lines JHU-SCC-011, JHU-SCC-013, and JHU-SCC-019 failed to support binding of the flowing lymphocytes. For comparison, we assessed lymphocyte binding to KG1a (human leukemia) cells and LS174T (colon cancer) cells as reported previously, given their known activity for L-selectin-mediated binding (18, 22). Both cell lines supported lymphocyte rolling at shear stresses between 0.6 and 4.0 dynes/cm2 (data not shown). Having estimated lymphatic shear to be 0.08 dynes/cm2, we next assayed adherent interactions at lower shear stresses (<0.6 dyne/cm2). The lymphocyte bolus was introduced into the chamber, and flow was halted and allowed to equilibrate (i.e. cells in flow stopped moving). Flow was then re-started at 0.01 dynes/cm2 and incrementally increased every 30 s up to a shear stress of 20 dynes/cm2. These experiments revealed adhesive interactions consisting of tethering and transient rolling preceding firm adhesion at shear stresses below 0.6 dynes/cm2 between the flowing human lymphocytes and the HNSCC cells, but not KG1a cells or LS174T cells (Fig. 1A). The HNSCC-lymphocyte adhesive interactions were observed between shear stresses of 0.035–0.9 dynes/cm2, with maximal activity at a wall shear stress of 0.07–0.08 dynes/cm2. A shear threshold was consistently observed at 0.035 dynes/cm2, and, importantly, no binding was observed under static conditions.
FIGURE 1.
HNSCC cells support binding to lymphocytes at shear stresses consistent with lymphatic flow. A, lymphocytes were first introduced at 0.5 ml/min into the parallel plate flow chamber containing monolayers of cancer cells. Flow was then stopped, followed by incremental increases in flow rate. JHU-SCC-011, JHU-SCC-013, and JHU-SCC-019 cells grown in a monolayer display peak shear-dependent interaction with fluid phase normal unfixed human lymphocytes at 0.07 and 0.08 dynes/cm2, respectively. KG1a and LS174T cells support binding of lymphocytes at shear stress levels consistent with venular flow (greater than 0.6 dynes/cm2) but not within the lower shear stress levels that support the interaction between the HNSCC cell lines and lymphocytes. * represents statistically significant difference from KG1a and LS174T cells at each corresponding shear stress. B, L-selectin mediates lymphocyte binding to HNSCC cells under low shear stress. The lymphocyte-JHU-SCC-019 interaction at 0.07 dynes/cm2 was reduced by 50% by pretreatment of lymphocytes in flow with LAM1-116 or anti-L-selectin polyclonal serum, all known to functionally block L-selectin-mediated binding at venular shear stresses. L-selectin was confirmed as important for the established low shear-dependent interaction by PMA pretreatment of lymphocytes in flow. The latter treatment is known to cause L-selectin shedding and integrin activation. No blocking was seen with preincubation with isotype antibody, rabbit nonimmune serum, or FBS. Error bars represent the mean ± S.E. * represents statistically significant difference from untreated. # represents statistically significant difference from PMA-treated.
Lymphocyte-HNSCC Cell Interaction Is Mediated by L-selectin—In initial studies, we sought to determine whether binding was affected by treatment of lymphocytes with PMA, a lymphocyte-activating agent known to cause L-selectin shedding and concomitant activation of integrin binding activity (25–27). Treatment of lymphocytes with PMA for 30 min at 37 °C resulted in a marked decrease in L-selectin expression (<5% of treated cells were L-selectin+ by flow cytometry). Using the parallel plate chamber, adhesion of PMA-activated lymphocytes with the tumor cell monolayer at 0.07 dynes/cm2 was reduced by 80% (Fig. 1B), indicating a dominant effect of L-selectin receptor-ligand interaction(s) on lymphocyte-HNSCC adhesion. We then incubated lymphocytes with blocking anti-L-selectin antibodies LAM1-116 or polyclonal anti-L-selectin serum to directly test whether L-selectin mediated shear stress-resistant interactions between lymphocytes and HNSCC cells at 0.07 dynes/cm2. Antibody blockade of L-selectin using LAM1-116 or polyclonal anti-L-selectin serum reduced binding by >50% (Fig. 1B). Collectively, these data show that L-selectin is an important effector of lymphocyte binding to HNSCC cells.
To further elucidate the discrete contribution of L-selectin in capturing HNSCC cells under flow conditions, unfixed HNSCC cells were perfused at defined shear stresses over human L-selectin/Fc chimera adhered to a solid support. To assess the upper limit of shear that can support initial tethering interactions, we initiated flow at 1.4 dynes/cm2 and then subsequently lowered shear stress in defined increments every 30 s. As shown in Fig. 2A, significant adherence of HNSCC cells to immobilized L-selectin was observed at an upper limit of 0.14 dynes/cm2, with maximal binding seen as shear was lowered to 0.07–0.03 dynes/cm2. No additional cells bound as shear was decreased from 0.03 to 0.02 dyne/cm2 (Fig. 2A), indicating that initial L-selectin-mediated capture of HNSCC is optimal at shear ∼0.03 dyne/cm2. Interestingly, although there was a clear threshold shear requirement to initiate tethering, the previously bound HNSCC did not detach from the L-selectin/Fc chimera at 0.02 dynes/cm2, the lowest level of shear tested. In addition, HNSCC attachment was fully abrogated by treatment with EDTA as well as by proteolytic treatment of the HNSCC cells with bromelain. Altogether, these findings show that the interaction is calcium-dependent, as is required for all known L-selectin-selectin ligand interactions, and suggests that a glycoprotein(s), rather than a glycolipid(s), serves as the relevant low shear L-selectin ligand(s) on the HNSCC cells. To ensure that the observed binding activity was indeed mediated by the L-selectin moiety of the chimera, plates with adhered chimera were incubated with the blocking antibody LAM1-116 or anti-L-selectin polyclonal serum prior to the infusion of the cancer cell suspensions. Incubation with function blocking anti-L-selectin antibodies resulted in complete abrogation of binding activity. Incubation with isotype control antibody had no effect (Fig. 2B). To determine whether L-selectin ligand(s) displayed by HNSCC required sialic acid, glycosylation, or sulfation for binding to L-selectin (characteristic features of L-selectin ligands expressed on cell membranes (28)), we treated HNSCC with α2–3-neuraminidase or α2–3,6,8-neuraminidase, O-sialoglycoprotein endopeptidase, tunicamycin, or sodium chlorate, respectively. Treatment with sialidases, tunicamycin, or sodium chlorate led to a marked reduction in L-selectin chimera binding at 0.07 dynes/cm2 (Fig. 2C). Treatment with O-sialoglycoprotein endopeptidase or heparinase did not alter binding activity. These results suggest the novel ligand(s) here reported require sialylation, sulfation, and N-linked glycosylation for maximal activity.
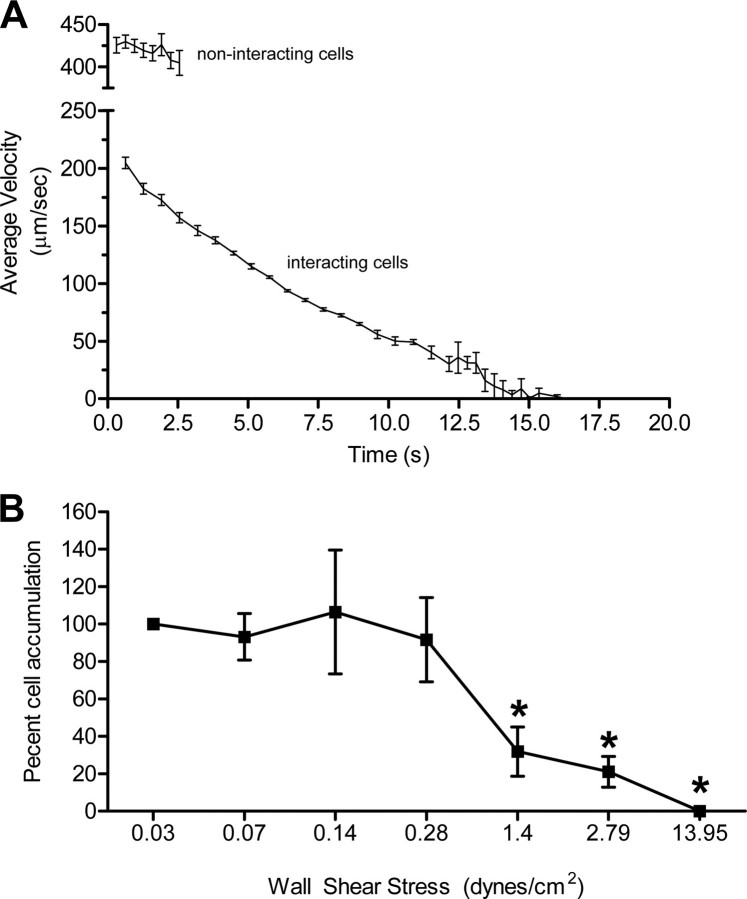
L-selectin-Selectin Ligand Interactions at Low Fluid Shear Display Elements of Rolling and Firm Adhesion—Our initial real time video characterization of this novel, low shear-based L-selectin binding activity suggested the interaction combined elements of rolling and firm adhesion. We thus further characterized the dynamics of binding by measuring interval displacement velocities exhibited by the JHU-SCC-019 cells interacting with adhered L-selectin chimera at 0.07 dynes/cm2. Interval velocity was calculated for individual cells by measuring the distance traveled by each cell within uniform successive intervals of 0.64 s. These measurements revealed a remarkably reproducible exponential decrease in velocity that asymptotically approached 0 μm/sec or firm adhesion (Fig. 3A). We further evaluated the shear resistance of the established L-selectin binding interaction by performing a detachment assay. In this assay, cancer cells were first introduced under low shear stress (0.07 dynes/cm2) over immobilized L-selectin chimera and allowed to bind. Shear stress was then incrementally increased while scoring the percentage of initial cells that remained bound at the end of each shear interval. These experiments revealed that, once the L-selectin interaction with the cancer cell was established, it was resistant to shear stresses as high as 1.4 dynes/cm2, 30-fold higher shear stress than required to establish the interaction (Fig. 3B). Notably, the cells displayed no forward displacement at the higher shear stresses, confirming firm adhesion. In contrast, KG1a cells and LS174T cells continuously rolled over adherent L-selectin chimera at 1 dyne/cm2, with an average rolling velocity of 18.0 μm/s (S.E. = 1.2 μm/s) and 30.8 μm/s (S.E. = 3.2 μm/s), respectively. The L-selectin-HNSCC cancer cell interaction thus displays elements of rolling and, once established, firm adhesion (Fig. 3, A and B).
FIGURE 3.
The cancer cell-L-selectin interaction supports tethering, rolling, and firm adhesion. A, JHU-SCC-019 cells were introduced in flow over human L-selectin/Fc chimera at 0.07 dynes/cm2. Experiments were recorded in real time, and rolling velocity was calculated by off-line image analysis of the forward displacement exhibited by interacting cells over successive intervals of 0.64 s. Similar analysis was performed on cells at the L-selectin chimera surface that were not interacting to measured hydrodynamic velocity. Once initial interaction was established between a cancer cell and the adhered L-selectin chimera (tethering), cells exhibited rolling as well as transition into firm adhesion. Measurements for the noninteracting cells were made over shorter time periods given the rapid transit of these free-flowing cells through the field of view. Error bars represent the mean ± S.E. B, interaction between cancer cells and L-selectin chimera is resistant to high shear stress once established under low shear. Cell-chimera interactions were first established at 0.07dynes/cm2 followed by sequential increases in fluid velocity (shear stress). Firm adhesion events at each discrete shear stress were normalized to the number of JHU-SCC-019 cells bound initially. Error bars represent the mean ± S.E. There was no statistically significant difference in binding at 0.03, 0.07, 0.14, and 0.28 dynes/cm2. Likewise, there was no statistically significant difference in binding at 1.4, 2.79, and 13.95 dynes/cm2. * represents statistically significant difference in binding compared with binding observed at 0.03, 0.07, 0.14, and 0.28 dynes/cm2.
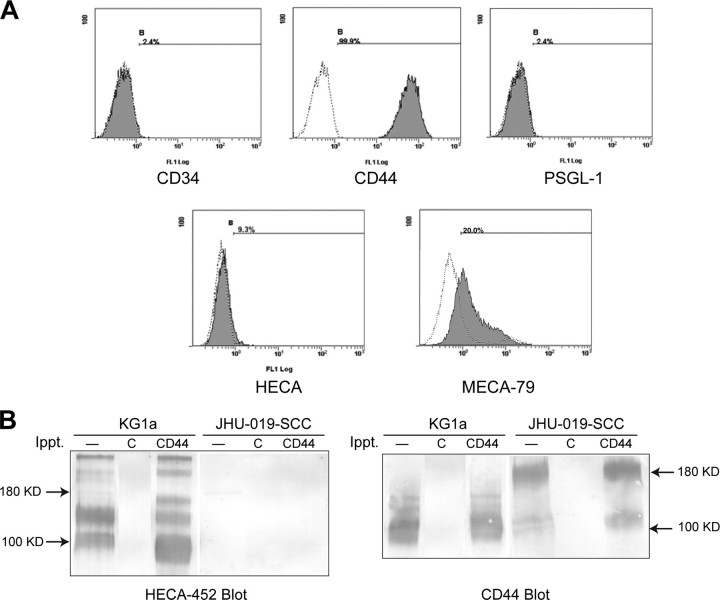
HNSCC Cells Express Novel L-selectin Glycoprotein Ligands Active under Conditions of Low Fluid Shear—By flow cytometry analysis, HNSCC cells displayed no HECA-452-reactive determinants (Fig. 4A). We evaluated HNSCC cells for expression of the three known human L-selectin glycoprotein ligands, PSGL-1, CD34, and a specialized sialofucosylated glycoform of CD44 known as HCELL (19, 29, 30). Flow cytometry and Western blot analysis showed that although the HNSCC cells express CD44, they lack HCELL, and they do not express PSGL-1 or CD34 (Fig. 4). Given the requirement for ligand sulfation for L-selectin binding (Fig. 2C), we examined HNSCC cells for the presence of sulfation-dependent MECA-79 antigens (28). By flow cytometry, a subpopulation of cells displayed MECA79 reactivity (see Fig. 4A). However, preincubating HNSCC cells with MECA-79 (at up to 50 μg/ml concentration) did not block binding of HNSCC cells to adhered L-selectin chimera at 0.07 dynes/cm2, whereas it did block lymphocyte binding to murine high endothelial venules in conventional Stamper-Woodruff assays (data not shown) (31).
FIGURE 4.
HNSCC cells express novel L-selectin ligands. A, expression of the three known L-selectin glycoprotein ligands, CD34, CD44 (HCELL), and PSGL1, as well as the carbohydrate determinants HECA-452 and MECA-79, was assessed on unfixed, native JHU-SCC-019 cells using flow cytometry. Staining with isotype antibody was used as negative control. No expression of CD34 or PSGL1 was detected; however, CD44 was found to be highly expressed. HECA-452 was not expressed, whereas MECA-79-reactivity was observed on a subpopulation of cells. B, to examine whether the specific sialofucosylated variant of CD44, HCELL, was expressed, CD44 was immunoprecipitated (Ippt.) from protein extracts made form JHU-SCC-019 cells and subjected to standard SDS-PAGE/Western blotting. The blot was then probed with the HECA-452 antibody that defines the sialofucosylated epitope characteristic of HCELL. No expression was detected on JHU-SCC-019 cells, whereas parallel experiments using KG1a cells, known to express HCELL, demonstrated robust expression.
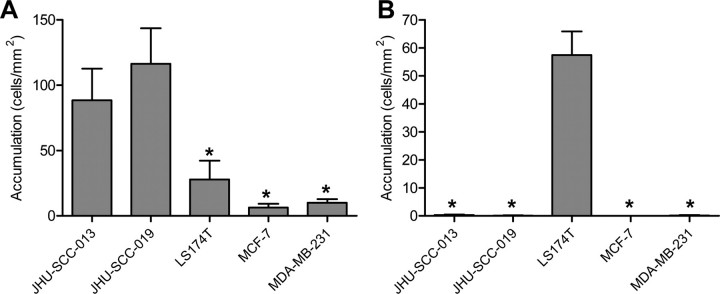
L-selectin-mediated Binding at Low Shear Stress Is a Distinctive Feature of HNSCC Cells—We next sought to determine whether L-selectin-mediated adherence of lymphocytes under low shear stress was a distinct feature of HNSCC cells compared with other solid malignancies. To this end, we examined JHU-SCC-013 (HNSCC), JHU-SCC-019 (HNSCC), LS174T (colon), MCF-7 (breast), and MDA-MB-231 (breast) cells for low shear L-selectin binding using the chimera-based assay. The HNSCC cells displayed significantly higher binding activity than any of the other cell lines at 0.07 dyne/cm2 (Fig. 5A). At 1 dyne/cm2, only LS174T cells adhered to L-selectin chimera as distinctly rolling interactions without firm adhesion (Fig. 5B).
FIGURE 5.
Shear-dependent L-selectin ligand activity varies by type of solid tumor. A, unfixed head and neck cancer, colon cancer, and breast cancer cell lines were assessed for low shear-dependent L-selectin binding by introducing the cancer cells in flow over adhered L-selectin/Fc chimera at 0.07 dynes/cm2. Head and neck cancer cells exhibited greater L-selectin ligand activity under conditions of lymphodynamic fluid shear. Error bars represent the mean ± S.E. * represents statistically significant difference from HNSCC cells. B, the same cell lines were examined for L-selectin ligand activity at 1 dyne/cm2. Only LS174T cells (colon) bound to adhered L-selectin chimera (rolling). Error bars represent the mean ± S.E. * represents statistically significant difference from LS174T cells.
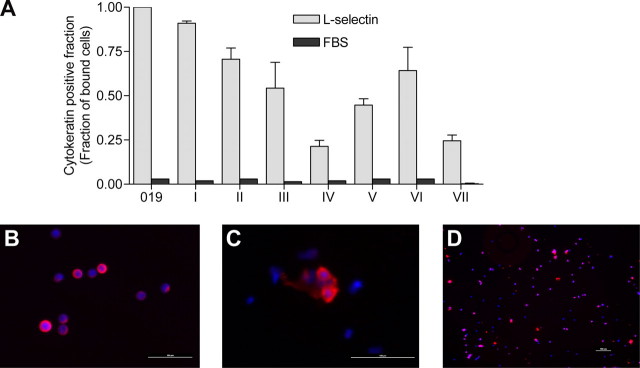
To determine whether the low shear stress-dependent L-selectin ligand activity observed on HNSCC cell lines is a phenotypic feature of cells within primary HNSCC tumor cells, we isolated cells from seven freshly resected native HNSCC tumor specimens for analysis. The cell suspension, derived from the center of the tumor mass, was introduced in flow over adherent L-selectin chimera at 0.07 dynes/cm2. Interacting cells were then fixed in situ by infusion of 4% paraformaldehyde through the chamber. The fixed cells were fluorescently stained for cytokeratin, a marker of squamous cell differentiation, to distinguish cancer cells from other non-cancer cells. Cytokeratin-positive cells from all seven primary tumors tested adhered to L-selectin chimera at 0.07 dynes/cm2 (Fig. 6). Binding was specific for L-selectin, as cytokeratin-positive tumor cells did not bind under flow conditions to surface treated with fetal bovine serum (FBS control) or to surface treated with human immunoglobulin (Ig control). Under flow, a variable number of cytokeratin-negative cells bound to adhered chimera, but such cells also bound to human Ig-treated surfaces, suggesting these interactions may be mediated by immunoglobulin receptors. No binding of cytokeratin-negative cells was observed on surface treated with FBS.
FIGURE 6.
Low shear stress-dependent L-selectin ligand activity is expressed on primary head and neck cancer cells. Primary cancer cells were obtained from seven tumors collected at the time of resection and introduced in flow over adherent L-selectin/Fc chimera at 0.07 dynes/cm2. Once binding was established, interacting cells were fixed in situ by infusion of 4% paraformaldehyde. Fixed cells were stained with a cytokeratin antibody to identify tumor cells. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. A, all primary tumors (i–vii) examined displayed L-selectin binding activity. Error bars represent the mean ± S.E. B, representative image of bound and fixed JHU-SCC-019 cells visualized by immunofluorescent staining for cytokeratin. C and D, representative picture of bound and fixed primary tumor cells (tumor 1) visualized by immunofluorescent staining for cytokeratin. Scale bars represent 100 μm.
DISCUSSION
The biology of selectin-selectin ligand interactions has been conventionally analyzed in the setting of hemodynamic fluid shear, as these molecules are important effectors of leukocyte homing in blood flow. In this context, selectins are primary mediators of a multireceptor cascade consisting of “tethering” contact of flowing cells onto the endothelium (selectins), cell rolling on endothelium (selectins), activation (chemokine receptors), and firm adhesion (integrins) followed by trans-endothelial migration of the engaged cells into the target interstitial space. Tethering is prerequisite for rolling, and, in general, rolling precedes firm adhesion. Rolling is represented by the rate of forward displacement of a cell stably attached to a substrate, measured as rolling velocity, below the hydrodynamic velocity of the fluid stream near the wall, which is estimated by measuring the velocity of cells in flow that fail to engage in adhesive interaction (3). In contrast, firm adhesion represents stable interaction without forward displacement. Notably for L-selectin, engagement to its respective ligand(s) is critically dependent on a threshold level of shear, below which the adhesive interaction is not stable (3). Prior studies showed that the shear stress range for L-selectin receptor-ligand interactions observed in vitro is equivalent to that found physiologically in the vasculature at post-capillary venules (3, 32–34); these studies have shown that L-selectin receptor-ligand interactions strictly function in promoting tethering/rolling with no inherent capacity to mediate firm adherence. Our findings here reveal an expanded biological role for L-selectin, which now includes mediating tethering-rolling interactions below typical hemodynamic shear stress levels, and, moreover, firm adhesion.
The data reported here provide the first evidence of a functional glycoprotein L-selectin ligand expressed on HNSCC cells. The L-selectin-dependent adhesion between lymphocytes and HNSCC cells mediates tethering and rolling interactions with a shear threshold requirement, consistent with prior models of L-selectin-dependent binding. However, the low levels of shear (0.03–0.2 dynes/cm2) supporting the initial HNSCC cell tethering on lymphocyte L-selectin in our study differ significantly from that reported previously for L-selectin receptor-ligand interactions (0.7–2 dynes/cm2) (3, 4). This low shear range for adhesion has been reported for β2 integrin-mediated tethering/rolling of activated human neutrophils to endothelial ICAM-1 in the absence of selectin contribution (35); however, contribution(s) by integrins is excluded here by evidence of HNSCC engagement to pure L-selectin chimera substrate. Interestingly, previous work has shown that in vitro chemical alteration of the known vascular L-selectin ligand CD34 alters its mechanical binding properties resulting in shear threshold-dependent binding to L-selectin over a lower shear stress range than that found in the native CD34 molecule (36). However, until now, no endogenous ligands have been identified that can mediate tethering-rolling interactions with L-selectin below ∼1 dyne/cm2 shear stress, such as is found within the lymphatic system. Furthermore, beyond the unique lymphodynamic range of engagement, the HNSCC cell L-selectin ligand(s) support L-selectin-dependent firm adhesion, as demonstrated by the detachment assay (Fig. 3B) showing no forward displacement of L-selectin-attached cells at shear stress up to 1.4 dynes/cm2. In particular, HNSCC cells were firmly adherent (no detectable forward displacement) at 1 dyne/cm2, in distinct contrast to KG1a (leukemia) cells and LS174T (colon cancer) cells that rolled on L-selectin/Fc chimera with average velocities of 18.0 ± 1.2 and 30.8 ± 3.2 μm/s, respectively. Altogether, our data show a unique “window” of shear stress engagement for L-selectin-dependent lymphocyte-HNSCC interactions. Yet once the initial interaction is established, adhesion will be sustained beyond the upper limit of shear permitting initial tethering interaction (Fig. 1A). Our findings thus reveal a new L-selectin-native L-selectin ligand interaction that displays behaviors expected of selectin-mediated binding (tethering/rolling, threshold shear requirement), as well as novel adhesion characteristics (low shear binding, firm adhesion), but all within a shear range below that previously defined for L-selectin-mediated adherence.
In addition to unique biophysical properties of L-selectin binding, several biochemical features distinguish the HNSCC ligand from that of all other previously described glycoprotein L-selectin ligands. Similar to lymph node HEV ligands and to PSGL-1, but unlike HCELL, the HNSCC L-selectin ligand displays sulfation-dependent L-selectin binding. However, similar to HCELL, but unlike PSGL-1 and conventional HEV L-selectin ligands, the HNSCC L-selectin binding determinant is displayed on N-glycans rather than O-glycans. Furthermore, although the precise structural features that direct binding activity for this ligand remain to be determined, blocking studies show that MECA79 antigen(s) do not comprise the relevant binding determinant on HNSCC cells. Notably, although L-selectin receptor-ligand interactions are the principal mediators of lymphocyte binding to HNSCC cells under low fluid shear, the results of anti-L-selectin blocking studies suggest that other receptor-ligand interactions may be contributory, and on-going studies are directed at identifying these relevant adhesion molecules. Altogether, these heretofore unrecognized effectors of low shear adhesive interactions warrant further study, not just for further elucidation of their unique biophysical and biochemical properties, but for understanding their role(s) in HNSCC tumor biology. The strong predilection for lymphatic metastases in head and neck cancer raises the possibility that the novel L-selectin receptor-ligand interactions described here may promote the process of lymph node lodgment of HNSCC tumor cells. A greater understanding of the HNSCC L-selectin ligand and of other effectors of HNSCC-lymphocyte binding under low fluid shear stress may lead to novel therapeutic modalities for a cancer whose survival statistics have remained unchanged for several decades.
Supplementary Material
Acknowledgments
We thank Dr. Cornelius Elferink, Dr. Lisa Elferink, Dr. Regino Perez-Polo, and Dr. Kathleen O'Connor for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL060528, RO1 HL073714, and RO1 CA121335 (to R. S.). This work was also supported by a National Research Service Award training grant (to M. M. B.). The authors have no conflict of interest disclosures. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental movies.
Footnotes
The abbreviations used are: HNSCC, head and neck squamous cell cancer; PBMC, peripheral blood mononuclear cells; PMA, phorbol 12-myristate 13-acetate; FBS, fetal bovine serum; PBS, phosphate-buffered saline; FBS, fetal bovine serum.
References
- 1.Kannagi, R. (2002) Curr. Opin. Struct. Biol. 12 599-608 [DOI] [PubMed] [Google Scholar]
- 2.Sackstein, R. (2005) Curr. Opin. Hematol. 12 444-450 [DOI] [PubMed] [Google Scholar]
- 3.Lawrence, M. B., Kansas, G. S., Kunkel, E. J., and Ley, K. (1997) J. Cell Biol. 136 717-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Springer, T. A. (1995) Annu. Rev. Physiol. 57 827-872 [DOI] [PubMed] [Google Scholar]
- 5.von Andrian, U. H., and Mempel, T. R. (2003) Nat. Rev. Immunol. 3 867-878 [DOI] [PubMed] [Google Scholar]
- 6.Brodt, P., Fallavollita, L., Bresalier, R. S., Meterissian, S., Norton, C. R., and Wolitzky, B. A. (1997) Int. J. Cancer 71 612-619 [DOI] [PubMed] [Google Scholar]
- 7.Burdick, M. M., McCaffery, J. M., Kim, Y. S., Bochner, B. S., and Konstantopoulos, K. (2003) Am. J. Physiol. 284 C977-C987 [DOI] [PubMed] [Google Scholar]
- 8.Gulubova, M. V. (2002) Histochem. J. 34 67-77 [DOI] [PubMed] [Google Scholar]
- 9.Skobe, M., Hawighorst, T., Jackson, D. G., Prevo, R., Janes, L., Velasco, P., Riccardi, L., Alitalo, K., Claffey, K., and Detmar, M. (2001) Nat. Med. 7 192-198 [DOI] [PubMed] [Google Scholar]
- 10.Mandriota, S. J., Jussila, L., Jeltsch, M., Compagni, A., Baetens, D., Prevo, R., Banerji, S., Huarte, J., Montesano, R., Jackson, D. G., Orci, L., Alitalo, K., Christofori, G., and Pepper, M. S. (2001) EMBO J. 20 672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He, Y., Kozaki, K., Karpanen, T., Koshikawa, K., Yla-Herttuala, S., Takahashi, T., and Alitalo, K. (2002) J. Natl. Cancer Inst. 94 819-825 [DOI] [PubMed] [Google Scholar]
- 12.Hoshida, T., Isaka, N., Hagendoorn, J., di Tomaso, E., Chen, Y. L., Pytowski, B., Fukumura, D., Padera, T. P., and Jain, R. K. (2006) Cancer Res. 66 8065-8075 [DOI] [PubMed] [Google Scholar]
- 13.Johnson, L. A., Clasper, S., Holt, A. P., Lalor, P. F., Baban, D., and Jackson, D. G. (2006) J. Exp. Med. 203 2763-2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forastiere, A., Koch, W., Trotti, A., and Sidransky, D. (2001) N. Engl. J. Med. 345 1890-1900 [DOI] [PubMed] [Google Scholar]
- 15.Fischer, M., Franzeck, U. K., Herrig, I., Costanzo, U., Wen, S., Schiesser, M., Hoffmann, U., and Bollinger, A. (1996) Am. J. Physiol. 270 H358-H363 [DOI] [PubMed] [Google Scholar]
- 16.Rocco, J. W., Leong, C. O., Kuperwasser, N., DeYoung, M. P., and Ellisen, L. W. (2006) Cancer Cell 9 45-56 [DOI] [PubMed] [Google Scholar]
- 17.Scher, R. L., Koch, W. M., and Richtsmeier, W. J. (1993) Arch. Otolaryngol. Head Neck Surg. 119 432-438 [DOI] [PubMed] [Google Scholar]
- 18.Burdick, M. M., Chu, J. T., Godar, S., and Sackstein, R. (2006) J. Biol. Chem. 281 13899-13905 [DOI] [PubMed] [Google Scholar]
- 19.Dimitroff, C. J., Lee, J. Y., Fuhlbrigge, R. C., and Sackstein, R. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 13841-13846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuhlbrigge, R. C., Alon, R., Puri, K. D., Lowe, J. B., and Springer, T. A. (1996) J. Cell Biol. 135 837-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanley, W. D., Burdick, M. M., Konstantopoulos, K., and Sackstein, R. (2005) Cancer Res. 65 5812-5817 [DOI] [PubMed] [Google Scholar]
- 22.Dimitroff, C. J., Lee, J. Y., Schor, K. S., Sandmaier, B. M., and Sackstein, R. (2001) J. Biol. Chem. 276 47623-47631 [DOI] [PubMed] [Google Scholar]
- 23.Sackstein, R., and Dimitroff, C. J. (2000) Blood 96 2765-2774 [PubMed] [Google Scholar]
- 24.Fuhlbrigge, R. C., King, S. L., Dimitroff, C. J., Kupper, T. S., and Sackstein, R. (2002) J. Immunol. 168 5645-5651 [DOI] [PubMed] [Google Scholar]
- 25.Chen, A., Engel, P., and Tedder, T. F. (1995) J. Exp. Med. 182 519-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung, T. M., and Dailey, M. O. (1990) J. Immunol. 144 3130-3136 [PubMed] [Google Scholar]
- 27.Kishimoto, T. K., Jutila, M. A., Berg, E. L., and Butcher, E. C. (1989) Science 245 1238-1241 [DOI] [PubMed] [Google Scholar]
- 28.Rosen, S. D. (2004) Annu. Rev. Immunol. 22 129-156 [DOI] [PubMed] [Google Scholar]
- 29.Puri, K. D., Finger, E. B., Gaudernack, G., and Springer, T. A. (1995) J. Cell Biol. 131 261-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spertini, O., Cordey, A. S., Monai, N., Giuffre, L., and Schapira, M. (1996) J. Cell Biol. 135 523-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sackstein, R., Fu, L., and Allen, K. L. (1997) Blood 89 2773-2781 [PubMed] [Google Scholar]
- 32.Diacovo, T. G., Puri, K. D., Warnock, R. A., Springer, T. A., and von Andrian, U. H. (1996) Science 273 252-255 [DOI] [PubMed] [Google Scholar]
- 33.Schramm, R., Schafers, H. J., Harder, Y., Schmits, R., Thorlacius, H., and Menger, M. D. (2006) Inflamm. Res. 55 160-167 [DOI] [PubMed] [Google Scholar]
- 34.Walcheck, B., Moore, K. L., McEver, R. P., and Kishimoto, T. K. (1996) J. Clin. Investig. 98 1081-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrence, M. B., Smith, C. W., Eskin, S. G., and McIntire, L. V. (1990) Blood 75 227-237 [PubMed] [Google Scholar]
- 36.Puri, K. D., Chen, S., and Springer, T. A. (1998) Nature 392 930-933 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.