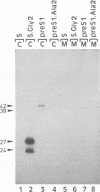
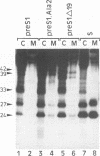
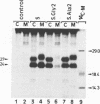
Abstract
The envelope of hepatitis B virus contains three related proteins, one of which is myristylated. The nonmyristylated small and middle protein are assembled into empty envelope particles which are secreted from cells, whereas the myristylated large envelope protein is mainly found in complete virions and is not secreted in the absence of the nucleocapsid. The block to secretion can be partially overcome by mutation or deletion of the myristylation site. Creation of a myristyl attachment site in the small protein impairs the secretion of empty envelope particles but not their intracellular assembly. Myristylation may therefore play a crucial role in hepatitis B virus replication by channeling the envelope proteins into complete viral particles.
Full text
PDF

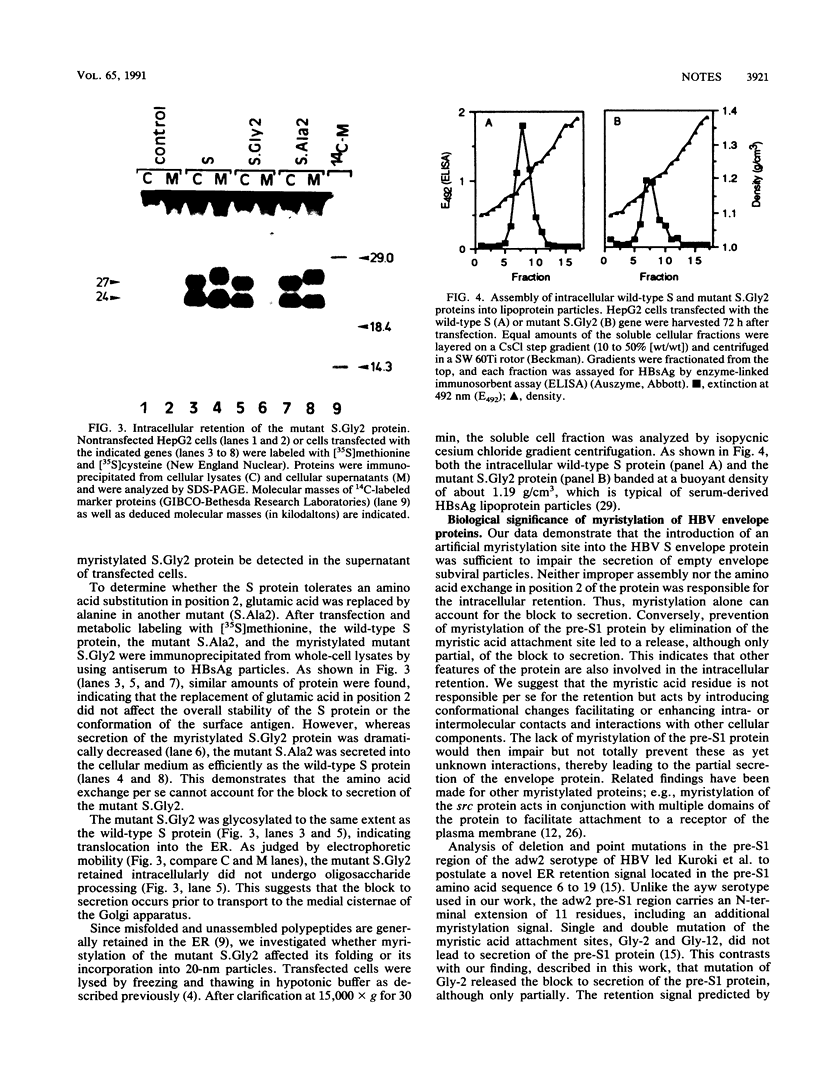


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Klopchin K., Moriyama T., Pasquinelli C., Dunsford H. A., Sell S., Pinkert C. A., Brinster R. L., Palmiter R. D. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989 Dec 22;59(6):1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989 Sep;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpeyroux F., Chenciner N., Lim A., Lambert M., Malpièce Y., Streeck R. E. Insertions in the hepatitis B surface antigen. Effect on assembly and secretion of 22-nm particles from mammalian cells. J Mol Biol. 1987 May 20;195(2):343–350. doi: 10.1016/0022-2836(87)90655-3. [DOI] [PubMed] [Google Scholar]
- Delpeyroux F., Peillon N., Blondel B., Crainic R., Streeck R. E. Presentation and immunogenicity of the hepatitis B surface antigen and a poliovirus neutralization antigen on mixed empty envelope particles. J Virol. 1988 May;62(5):1836–1839. doi: 10.1128/jvi.62.5.1836-1839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C. A., Reynolds P. L., Hruby D. E. Fatty acid acylation of vaccinia virus proteins. J Virol. 1989 Oct;63(10):4285–4291. doi: 10.1128/jvi.63.10.4285-4291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Israel N., Chenciner N., Houlmann C., Streeck R. E. An expression vector for high-level protein synthesis in Vero cells. Gene. 1989 Sep 30;81(2):369–372. doi: 10.1016/0378-1119(89)90198-4. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. M., Varmus H. E., Bishop J. M. The src protein contains multiple domains for specific attachment to membranes. Mol Cell Biol. 1990 Mar;10(3):1000–1009. doi: 10.1128/mcb.10.3.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzewicz N., Streuli C. H., Stuart-Smith N., Jones M. D., Wallace S., Griffin B. E. Myristylated polyomavirus VP2: role in the life cycle of the virus. J Virol. 1990 Sep;64(9):4414–4420. doi: 10.1128/jvi.64.9.4414-4420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Hölscher C., Reuer Q., Harber J., Wimmer E. Myristoylation of the poliovirus polyprotein is required for proteolytic processing of the capsid and for viral infectivity. J Virol. 1990 May;64(5):2433–2436. doi: 10.1128/jvi.64.5.2433-2436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki K., Russnak R., Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989 Oct;9(10):4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfalla G., Armbruster L., Dequin S., Bertolotti R. Construction of an EBNA-producing line of well-differentiated human hepatoma cells and of appropriate Epstein-Barr virus-based shuttle vectors. Gene. 1989 Mar 15;76(1):27–39. doi: 10.1016/0378-1119(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Marc D., Masson G., Girard M., van der Werf S. Lack of myristoylation of poliovirus capsid polypeptide VP0 prevents the formation of virions or results in the assembly of noninfectious virus particles. J Virol. 1990 Sep;64(9):4099–4107. doi: 10.1128/jvi.64.9.4099-4107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt O., Heermann K. H., Seifer M., Gerlich W. H. Cell-type dependent expression and secretion of hepatitis B virus pre-S1 surface antigen. Postgrad Med J. 1987;63 (Suppl 2):41–50. [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Kimber K. L., Jarocki-Witek V., Dheer S. K., Vernon S. K., Conley A. J., Davis A. R., Hung P. P. Distinctive properties of the hepatitis B virus envelope proteins. J Virol. 1988 Feb;62(2):407–416. doi: 10.1128/jvi.62.2.407-416.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Rutter W. J. Regulation of secretion of the hepatitis B virus major surface antigen by the preS-1 protein. J Virol. 1987 Mar;61(3):782–786. doi: 10.1128/jvi.61.3.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Nakamura G. R., Simonsen C. C., Levinson A. D., Brands R. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J Virol. 1986 Jun;58(3):884–892. doi: 10.1128/jvi.58.3.884-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Heat shock and the sorting of luminal ER proteins. EMBO J. 1989 Nov;8(11):3171–3176. doi: 10.1002/j.1460-2075.1989.tb08475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science. 1986 Dec 12;234(4782):1388–1391. doi: 10.1126/science.3787251. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J Virol. 1987 May;61(5):1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. Specific and saturable binding of pp60v-src to plasma membranes: evidence for a myristyl-src receptor. Cell. 1989 Jul 28;58(2):281–286. doi: 10.1016/0092-8674(89)90842-8. [DOI] [PubMed] [Google Scholar]
- Sells M. A., Zelent A. Z., Shvartsman M., Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol. 1988 Aug;62(8):2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., Lingappa V. R., Ganem D. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J Cell Biol. 1988 Dec;107(6 Pt 1):2163–2168. doi: 10.1083/jcb.107.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly J., Howard C. R., Zuckerman A. J. Hepatitis B polypeptide vaccine preparation in micelle form. Nature. 1981 Mar 5;290(5801):51–54. doi: 10.1038/290051a0. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Rutter W. J. Assembly of viral particles in Xenopus oocytes: pre-surface-antigens regulate secretion of the hepatitis B viral surface envelope particle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9338–9342. doi: 10.1073/pnas.83.24.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C. H., Griffin B. E. Myristic acid is coupled to a structural protein of polyoma virus and SV40. Nature. 1987 Apr 9;326(6113):619–622. doi: 10.1038/326619a0. [DOI] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]