Abstract
CULLIN 2 (CUL2) is a component of the ElonginB/C-CUL2-RBX-1-Von Hippel-Lindau (VHL) tumor suppressor complex that ubiquitinates and degrades hypoxia-inducible factor α (HIFα). HIFα is a transcription factor that mediates the expression of hypoxia-sensitive genes, including vascular endothelial growth factor (VEGF), which in turn regulates vasculogenesis. Whereas CUL2 participates in the degradation of HIFα, the potential role of CUL2 in the regulation of other cellular processes is less well established. In the present study, suppression of CUL2 expression by Cul2 siRNA inhibited HIFα transcriptional activation of the VEGF gene in vitro, indicating that CUL2 plays a role distinct from its known function in HIFα degradation. Because ARNT heterodimerizes with HIFα, we assessed whether CUL2 influenced ARNT expression. Cul2 siRNA inhibited the expression of endogenous ARNT. Ectopically expressed ARNT reversed the inhibition of HIF activity by Cul2 siRNA in the VEGF promoter, suggesting that CUL2 regulates HIF activation through ARNT. In 786-O cells lacking VHL, Cul2 siRNA suppressed the expression of both ARNT and VEGF, indicating that CUL2 regulates HIF activity independently of VHL. In transgenic zebrafish expressing GFP driven by the Flk promoter (a known HIF target), zCul2 morpholino blocked embryonic vasculogenesis in a manner similar to that caused by inhibition of VEGF-A. In the zebrafish embryos, zCul2 inhibited the expression of CUL2, VEGF, and Flk-GFP protein, indicating that CUL2 is required for expression of other vasculogenic HIF targets. Taken together, CUL2 is required for normal vasculogenesis, at least in part mediated by its regulation of HIF-mediated transcription.
CULLIN 2 (CUL2)2 is a member of the CULLIN family of ubiquitin ligases (1). CUL2 associates with the von Hippel-Lindau tumor suppressor protein (VHL), transcriptional elongation factors Elongin B/C, RING-box protein RBX1, and E3 ubiquitin-protein ligase (2-5). VHL recognizes hydroxylated hypoxia-inducible factor α (HIFα), recruits the CUL2-associating complex on HIFα, and ubiquitinates HIFα (6-11). The ubiquitinated HIFα is destined for degradation. In the absence of CUL2 or RBX1, the HIF-2α protein is increased, indicating that CUL2 and RBX1 are involved in HIFα protein stabilization (12). CUL2, together with ElonginB/C and RBX1, also forms a complex with MED8, a mediator subunit of the RNA polymerase II transcriptional machinery (13), though the mechanism by which CUL2 influences transcriptional regulation is unknown. CUL2 and RBX1 have been predicted to function as tumor suppressor proteins because of their protein-protein interaction with VHL. Somatic mutations in VHL have been associated with tumors including hemangioblastomas, renal cell carcinoma, and pheochromocytoma (14). However, pathogenic mutations in the Cul2 or Rbx1 gene causing carcinoma have not been identified (14-16), indicating that CUL2 or RBX1 may not be tumor suppressor proteins. In fact, CUL2 functions as a positive cell cycle regulator in Caenorhabditis elegans (17). Other than its interaction with VHL, the biological and molecular functions of CUL2 are not well understood.
HIF-1α and HIF-2α (HIFα) are transcription factors that heterodimerize with ARNT to regulate vasculogenesis, angiogenesis, glucose metabolism, and erythropoiesis. HIFα regulates the activity of numerous genes, including VEGF, glucose transporter, erythropoietin, and others (18). Whereas HIF-1α is generally hypoxia-responsive and under the control of the ElonginB/C-CUL2-RBX-1-VHL ubiquitination/degradation complex, the HIF-2α protein is expressed under more diverse conditions, including normoxia (19-21).
In the present study, suppression of CUL2 by Cul2 siRNA induced HIFα protein levels. Surprisingly, HIFα-mediated VEGF transcription was concomitantly decreased, indicating that CUL2 is required for HIFα activity. RBX1, another component of CUL2-associating proteins, was also required for HIFα activity, serving a role in the stabilization of CUL2. Reduction of CUL2 reduced ARNT expression, resulting in the decrease of HIFα activity on the VEGF promoter. Ectopically expressed ARNT reversed the inhibitory effect of CUL2 on VEGF transcription, further indicating that CUL2 regulates HIFα activity through ARNT. Suppression of CUL2 by zCul2 morpholino in zebrafish abrogated embryonic development and caused vascular defects. Whereas CUL2 is a component of the VHL ubiquitination/degradation complex, it also participates in HIFα-mediated regulation of vasculogenesis.
EXPERIMENTAL PROCEDURES
Plasmids, Expression Constructs, siRNAs, and Chemicals—The human HIF-2α expression vector pcDNA3.HIF-2α was a gift from Dr. Richard K. Bruick (22). ARNT and FLAG-tagged ARNT expression vectors (pcDNA3.ARNT and FLAG hARNT) were made by cutting human ARNT cDNA out from pBM5/Neo/D24-1 (a gift from Dr. Oliver Hankinson) (23) using HindIII sites and inserting into the pcDNA3.1 vector and pCMV-3Tag-1 vector (FLAG vector). The expression vector containing the FLAG-tagged human HIF-2α C terminus (amino acids 495-870; activation domain) (p3xFLAG-CMV-7.1.HIF-2C, FLAG-HIF-2α) was made by inserting PCR products from pcDNA3.HIF-2α. The luciferase reporter constructs containing the 5′-flanking region of the human VEGF promoter (pGL2.hVEGF, 2.6 kb; hVEGF-Luciferase) and the human KDR/flk promoter (pGL2.-4kb+296.KDR/flk-1; hFlk-1-Luciferase) were gifts from Dr. Debabrata Mukhopadhyay (24) and Dr. Cam Patterson (25). All siRNAs targeting Cul2 no. 1 (ID: 139191), Cul2 no. 2 (ID: 139190), Rbx1 (ID: 20973), Tip49 (ID: 13702), ARNT (ID: 106535), and negative control siRNA were purchased from Ambion (Austin, TX). Deferoxamine mesylate (DFO) was purchased from Sigma.
Transient Transfection Assays—Transient transfection reporter assay was performed as described previously (26) except that hVEGF-Luciferase and hFlk-1-Luciferase reporter constructs and CMV.β-galactosidase were added to each well using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Transfection for siRNAs was performed according to protocols using Lipofectamine 2000 for H441 and HeLa cells and Lipofectamine RNAiMAX for 786-O cells provided by Invitrogen. Relative light units were obtained by the mean ± S.D. of the luciferase activity normalized to β-galactosidase activity from triplicate wells. The results from one of at least three representative experiments are given as the mean of fold activation. Error bars indicate the S.D.
Immunoblot and Co-immunoprecipitation Assays—Immunoblot and Co-immunoprecipitation assays were performed as described previously (26) except that membranes were incubated with anti-CUL2 (Zymed Laboratories Inc., San Francisco, CA), anti-ARNT (Alexis, San Diego, CA), anti-HIF-2α, anti-HIF-1α (Novus Biologicals, Littleton, CO), anti-β-actin, anti-FLAG (Sigma), anti-RBX1 (BIOSOURCE, Camarillo, CA), anti-TIP49 (ProteinTech Group, Chicago, IL), anti-TIP60 (Upstate, Lake Placid, NY), anti-GAPDH (Chemicon-Millipore, Billerica, MA), and anti-zebrafish VEGF (R&D Systems, Minneapolis, MN).
Quantitative RT-PCR—Total RNA was extracted with the RNeasy mini kit (Qiagen, Valencia, CA), and cDNA was synthesized with Superscript III reverse transcriptase kit according to the manufacturer's protocol (Invitrogen). A 7300 Real Time PCR System (Applied Biosystems, Foster City, CA) was used for quantitative PCR, with TaqMan probes from Applied Biosystems. Data of each sample were normalized to the expression of endogenous control gene (18 S rRNA). The relative expression of each target gene is expressed as the fold change relative to that of control siRNA-treated cells.
Immunoprecipitation and Protein Identification by Mass Spectrometry—The pull-down assay was performed as described previously (26). Briefly, FLAG-HIF-2α was transfected in MLE-15 mouse lung epithelial cells. After 24 h, the transfected cells were harvested. The cell lysates were incubated with anti-FLAG M2 antibody attached to agarose beads (Sigma) for 16 h at 4 °C and eluted with 3×FLAG peptide (Sigma) after the beads were washed, according to the manufacturer's instructions. The eluted proteins were boiled in SDS sample loading buffer, fractionated by SDS-PAGE, and detected with Coomassie Blue staining. Protein identification was performed using mass spectrometry described previously (27).
Immunocytochemistry—HeLa, H441 and 786-O cells were seeded in chamber slides (NUNC). Before staining, cells were washed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. After fixation, cells were washed with PBS, and blocked with 4% goat serum in PBS with Triton X-100 (PBST) for 1 h at room temperature. The CUL2 antibody (Abcam, Cambridge, MA, 1:100 diluted in PBST containing 4% goat serum) and the VHL antibody (BD Pharmingen, San Jose, CA, 1:1000 diluted in PBST containing 4% goat serum) were incubated overnight at 4 °C. After washing, secondary antibodies were added, and the slides were counterstained with DAPI mounting medium (Vector Laboratory, Burlingame, CA).
Zebrafish Maintenance—Flk1:egfp transgenic zebrafish (Flk-GFP) were kindly provided by Dr. Didier Stainier (28). The transgenic zebrafish and Tübingen Long Fin (TL) strain wild-type zebrafish were maintained with a 10/14 h dark/light photoperiod and a temperature of 26.5-28.5 °C.
Morpholino Design and Microinjection—An antisense morpholino against the zebrafish Cul2 gene (gene ID: ENSDARG00000013965) was designed and synthesized by Gene-Tools, Inc. The oligo sequence (5′-GGA CAT GGT GTG TGG CTT TTT TTTC-3′) blocks the translation start codon (underlined). The morpholino was prepared in ddH2O (10 mg/ml), diluted with Danieu's buffer and mixed with phenol red to a final concentration of either 0.5 μg/μl or 1 μg/μl prior to injection. Heterozygous flk1:egfp transgenic eggs or TL wild-type eggs were injected with the morpholinos (1.15-6.9 ng) at the 1-cell stage. Eggs were maintained in egg water at 28.5 °C. Embryos were photographed with a LEICA MZ 16FA stereo fluorescent microscope and LEICA DFC 480 digital camera.
Statistical Analyses—Statistical differences were determined using unpaired 2-tailed Student's t tests. A p value of less than 0.05 (indicated as a single asterisk, *) was considered to be significant.
RESULTS
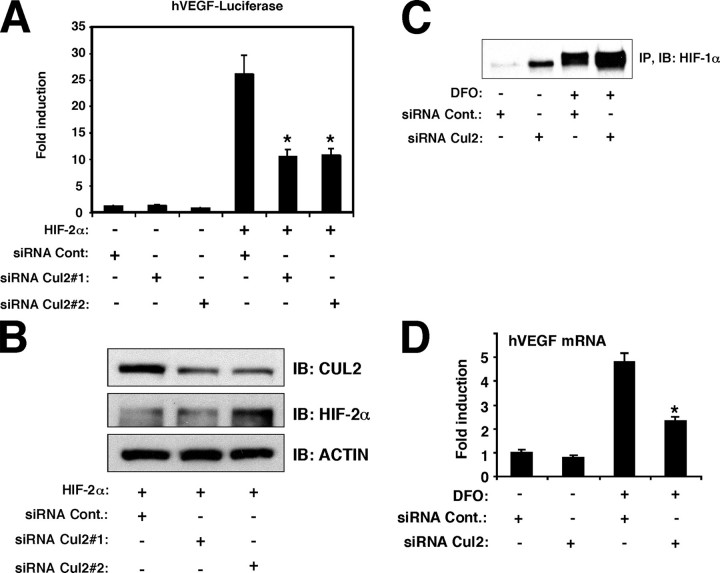
Cul2 siRNA Suppressed HIFα-mediated Activation of VEGF—CUL2 influences ubiquitination and degradation of HIF-2α via the ElonginB/C-CUL2-RBX-1-VHL ubiquitination/degradation complex (12). Under normoxic conditions, ectopically expressed HIF-2α, but not HIF-1α, is translocated into the nucleus, stimulating expression of hypoxia-inducible genes (19). To test whether CUL2 influences the transcriptional activity of HIF-2α, the effect of Cul2 siRNA was measured on HIF-2α-dependent activity of the VEGF promoter in H441 lung cancer cells. HIF-2α stimulated the VEGF promoter under normoxic conditions. Unexpectedly, the stimulatory activity of HIF-2α on the VEGF promoter was suppressed in the presence of two distinct Cul2 siRNAs that target the expression of the Cul2 gene (no. 1; Exon 4 and no. 2; Exon 2) (Fig. 1A). CUL2 proteins were decreased in the presence of either Cul2 siRNAs, while ectopically expressed HIF-2α protein was little increased or not altered. Endogenous β-actin was not influenced by Cul2 siRNAs (Fig. 1B). These results indicate that CUL2 is required for HIF-2α transcriptional activity of the VEGF promoter.
FIGURE 1.
CUL2 is required for HIFα-dependent activation of the VEGF promoter. A, CUL2 siRNA inhibited HIF-2α activity. H441 cells were transfected with 1 μg of hVEGF luciferase construct (hVEGF-Luciferase), 1 μg of pCMV.β-galactosidase, 1 μg of HIF-2α expression vector (pcDNA3.1.HIF-2α), 100 pmol of siRNA negative control (siRNA Cont.), or Cul2-targeting siRNA (siRNA Cul2) as indicated. The cells were incubated for 48 h before harvest. Results are presented as fold activation of light units normalized to β-galactosidase activity relative to control constructs. B, Cul2 siRNA effectively suppressed CUL2 protein expression. Cell lysates prepared in A were blotted with anti-CUL2 and anti-HIF-2α to identify CUL2 and HIF-2α. Anti-β-actin was used as a negative control. C, Cul2 siRNA stabilized the HIF-1α protein. HeLa cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.) or Cul2-targeting siRNA (siRNA Cul2#1) as indicated. 24 h after transfection, 300 μm DFO was added to the transfected cells and incubated for 30 h before cell harvest. The immunoprecipitates obtained with anti-HIF-1α antibody (IP) were separated by SDS-PAGE, transferred to the membrane, and blotted with anti-HIF-1α (IB) as described under “Experimental Procedures.” D, Cul2 siRNA inhibited DFO-mediated VEGF induction. HeLa cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.) or Cul2-targeting siRNA (siRNA Cul2#1) as indicated. 24 h after transfection, 300 μm DFO was added, and the cells were incubated for 30 h before cell harvest. Results of real-time PCR are presented as fold activation of VEGF mRNA normalized to 18 S rRNA.
To assess the effect of CUL2 on the transcriptional activity of endogenous HIFα, the activity of the endogenous HIF-1α induced by an iron chelator deferoxamine DFO, a hypoxia mimetic agent, was measured in the presence/absence of Cul2 siRNA in HeLa cells (Fig. 1, C and D). VEGF transcription is known to be mediated by HIF-1α rather than HIF-2α in HeLa cells (29). As expected, Cul2 siRNA induced endogenous HIF-1α protein, likely through disruption of the VHL protein degradation complex (Fig. 1C), consistent with the known role of CUL2 in HIFα degradation (12). DFO also induced the amount of HIF-1α protein. In the presence of both Cul2 siRNA and DFO, the HIF-1α protein was further induced (Fig. 1C). DFO induced the endogenous VEGF mRNA in HeLa cells as reported previously (Ref. 30 and Fig. 1D). Despite increased HIF-1α, VEGF mRNA induced by DFO was decreased in the presence of Cul2 siRNA (Fig. 1D), consistent with its effect on the VEGF promoter activity in Fig. 1A. Though CUL2 participates in the degradation of HIFα protein, the loss of CUL2 unexpectedly suppressed HIFα transcriptional activity.
RBX1-CUL2 but Not TIP49 Is Required for HIF-2α Activity—CUL2 forms a protein complex with the RING-box protein RBX1 (9). To determine the effects of RBX1 on the CUL2 protein and regulation on HIFα transcription, RBX1 expression was suppressed by Rbx1 siRNA. In the presence of Rbx1 siRNA, both RBX1 and CUL2 proteins were decreased. In contrast, Cul2 siRNA did not alter RBX1 protein levels (Fig. 2A), suggesting that RBX1 modulates CUL2 protein stability. Suppression of RBX1 protein expression by Rbx1 siRNA inhibited transcriptional activity of HIF-2α, perhaps through the suppression of the CUL2 protein (Fig. 2B). Thus, RBX1 is required for the maintenance of CUL2 levels and HIF-2α activation of the VEGF promoter.
FIGURE 2.
RBX1-CUL2 is required for HIF-2α-mediated activation of the VEGF promoter. A, Rbx1 siRNA suppressed RBX1 and CUL2 protein expression. HeLa cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.), Cul2-targeting siRNA (siRNA Cul2#1), or Rbx1-targeting siRNA (siRNA Rbx1). Cell lysates were blotted with anti-RBX1 and anti-CUL2 to identify RBX1 and CUL2. Anti-β-actin was used as a negative control. B, Rbx1 siRNA suppressed HIF-2α activity. H441 cells were transfected with 1 μg of the hVEGF luciferase construct (hVEGF-Luciferase), 1 μg of pCMV.β-galactosidase, 1 μg of HIF-2α expression vector (pcDNA3.1.HIF-2α), 100 pmol of siRNA negative control (siRNA Cont.), or Rbx1-targeting siRNA (siRNA Rbx1) as indicated. Results are presented as fold activation of light units normalized to β-galactosidase activity relative to control constructs. C, HIF-2α bound to TIP49 but not TIP60. Immunoprecipitates (IP) were prepared from MLE-15 (mouse lung epithelial cells) after transfection with FLAG-HIF-2α or FLAG empty control vector. The immunoprecipitates obtained with anti-FLAG antibody (IP: FLAG) were separated by SDS-PAGE, transferred to the membrane, and blotted with anti-FLAG, anti-TIP49, and anti-TIP60 as described under “Experimental Procedures.” D, both Cul2 siRNA and Tip49 siRNA were target-specific. HeLa cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.), Cul2-targeting siRNA (siRNA Cul2#1), or Tip49-targeting siRNA (siRNA Tip49). Cell lysates were blotted with anti-CUL2 and anti-TIP49 to identify CUL2 and TIP49. *, nonspecific band. Arrow, TIP49-specific band. E, Tip49 did not influence DFO-mediated VEGF transcription. HeLa cells were transfected with 1μg of the hVEGF luciferase construct (hVEGF-Luciferase), 1μg of pCMV.β-galactosidase, 100 pmol of siRNA negative control (siRNA Cont.), Cul2-targeting siRNA (siRNA Cul2#1), or Tip49-targeting siRNA (siRNA Tip49) as indicated. 24 h after transfection, 300 μm DFO was added to the transfected cells and incubated for 30 h before cell harvest. Results are presented as fold activation of light units normalized to β-galactosidase activity relative to control constructs.
To further identify proteins required for transcriptional activation by HIFα, HIF-2α with a FLAG tag of the C terminus activation domain (FLAG-HIF-2α) (31) was used to co-precipitate proteins from extracts of mouse lung epithelial (MLE) cells that were then identified by mass spectrometry (data not shown) and subsequent immunoblotting. TATA box-binding protein-interacting protein, 49-kDa (TIP49) was identified as a protein that co-immunoprecipitated with FLAG-HIF-2α (Fig. 2C). TIP49 was identified as an essential cofactor for the oncoprotein c-Myc and β-catenin (32, 33). TIP49 is a part of the TIP60 (TAT-interacting protein, 60-kDa) complex that has histone acetyltransferase activity (34). TIP60 was not detected in the HIF-2α co-immunoprecipitates. The interaction between TIP49 and HIF-2α indicated that TIP49 might be a co-activator for HIF-2α (Fig. 2C). Tip49 siRNA selectively reduced TIP49 protein expression, but did not affect CUL2 protein levels. Conversely, Cul2 siRNA did not affect TIP49 protein levels (Fig. 2D), indicating that both siRNAs were target-specific. Whereas Cul2 siRNA inhibited VEGF transcription induced by DFO, Tip49 siRNA did not influence DFO-dependent VEGF transcription (Fig. 2E), suggesting that CUL2, but not TIP49, is required for HIF-1α-mediated VEGF transcription.
CUL2 Influences HIF-2α Transcriptional Activity through ARNT—ARNT heterodimerizes with HIFα and is required for HIFα transcriptional activity (18). Consistent with this concept, HIF-2α activity on the VEGF promoter was suppressed in the presence of ARNT siRNA that reduced ARNT protein levels (Fig. 3, A and B). The CUL2 protein was only marginally influenced by ARNT siRNA. The β-actin protein was not altered by ARNT siRNA (Fig. 3A). As expected, the loss of ARNT inhibited the transcriptional activity of HIF-2α in the VEGF promoter (Fig. 3B).
FIGURE 3.
CUL2 influences HIF-2α activity through ARNT. A, ARNT siRNA suppressed ARNT protein expression. H441 cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.) or ARNT-targeting siRNA (siRNA ARNT). Cell lysates were blotted with anti-ARNT and anti-CUL2 to identify ARNT and CUL2. Anti-β-actin was used as a negative control. B, ARNT siRNA inhibited HIF-2α activity. H441 cells were transfected with 1 μg of the hVEGF luciferase construct (hVEGF-Luciferase), 1 μg of pCMV.β-galactosidase, 1 μg of HIF-2α expression vector (pcDNA3.1.HIF-2α), 100 pmol of siRNA negative control (siRNA Cont.), or ARNT-targeting siRNA (siRNA ARNT) as indicated. Results are presented as fold activation of light units normalized to β-galactosidase activity relative to control constructs. C, Cul2 siRNA suppressed CUL2 and ARNT protein expression. HeLa cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.) or Cul2-targeting siRNA (siRNA Cul2#1). Cell lysates were blotted with anti-CUL2 and anti-ARNT to identify CUL2 and ARNT. Anti-β-actin was used as a negative control. D, Cul2 siRNA suppressed ARNT mRNA expression. HeLa cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.) or Cul2-targeting siRNA (siRNA Cul2#1) as indicated. The cells were incubated for 48 h before cell harvest. Results of real-time PCR are presented as fold activation of ARNT mRNA normalized to 18 S rRNA. E, ectopically expressed ARNT reversed the effect of Cul2 siRNA on HIF-2α activity. H441 cells were transfected with 1 μg of the hVEGF luciferase construct (hVEGF-Luciferase), 1 μg of pCMV.β-galactosidase, 1 μg of HIF-2α expression vector (pcDNA3.1.HIF-2α), 1 μg of ARNT expression vector (pcDNA3.1ARNT), 100 pmol of siRNA negative control (siRNA Cont.), or Cul2-targeting siRNA (siRNA Cul2#1) as indicated. Results are presented as fold activation of light units normalized to β-galactosidase activity relative to control constructs.
In the absence of CUL2, ARNT protein expression was suppressed (Fig. 3C). ARNT mRNA was also suppressed by Cul2 siRNA (Fig. 3D). To assess whether CUL2 regulates HIF activity through the inhibition of ARNT, the effects of Cul2 siRNA on HIF-2α activity were measured in the presence of ectopically expressed ARNT. At baseline, ectopically expressed ARNT, along with HIF-2α, further induced the VEGF promoter activity, indicating that ARNT is a limiting factor in H441 cells. Cul2 siRNA strongly inhibited HIF-2α activity on the VEGF promoter, and ectopically expressed ARNT significantly reversed the inhibitory effects of Cul2 siRNA (Fig. 3E), indicating that CUL2 influences HIF-2α transcriptional activity, at least in part by regulating ARNT expression.
CUL2 Regulates VEGF mRNA Production Independently of VHL—CUL2 affects protein stability of HIF-1α (Fig. 1C), likely through interactions with the VHL ubiquitination/degradation complex as previously reported (12). Our results indicated that CUL2 regulates HIFα activity independently of HIFα protein stability (Fig. 3). To test whether VHL is involved in the CUL2 regulation of VEGF mRNA production, the VEGF mRNA production was measured in 786-O VHL-defective renal cells in the presence/absence of CUL2. In these cells, CUL2 was effectively suppressed by Cul2 siRNA. ARNT was also suppressed by Cul2 siRNA (Fig. 4A), similar to previous results in HeLa cells (Fig. 3C). In contrast to other cells, HIF-2α is endogenously expressed in 786-O cells because of the VHL defect (35). Unexpectedly, endogenous HIF-2α was decreased or not altered by Cul2 siRNA in 786-O cells (Fig. 4, A and C), contrasting with the finding that endogenous HIF-1α was induced by Cul2 siRNA in HeLa cells (Fig. 1C), indicating distinct regulation of HIFα by CUL2 in 786-O cells. ARNT mRNA and VEGF mRNA were inhibited by Cul2 siRNA in 786-O cells (Fig. 4B, upper panels and Fig. 4D), consistent with a role for CUL2 in the maintenance of ARNT protein expression by a process independent from VHL (Figs. 3 and 4A). The effectiveness of the siRNAs was validated in 786-O cells, wherein ARNT siRNA suppressed ARNT mRNA and VEGF mRNA (Fig. 4B, lower panels). Ectopically expressed ARNT did not reverse the inhibitory effects of Cul2 siRNA on the VEGF mRNA in 786-O cells (Fig. 4D), a finding distinct from the observation that ARNT overexpression in H441 cells reversed the inhibitory effect of Cul2 siRNA (Fig. 3E). These results indicate that Cul2 siRNA suppresses CUL2 intermediate factors other than ARNT in 786-O cells and thus, ectopically expressed ARNT is not sufficient to reverse the effect of Cul2 siRNA. Taken together, CUL2 influences HIF-mediated transcription independently of VHL, and CUL2 regulates VEGF gene expression through CUL2 intermediate factors in addition to ARNT.
FIGURE 4.
CUL2 is required for ARNT and VEGF mRNA expression in 786-O (VHL defective) cells. A, Cul2 siRNA suppressed CUL2, ARNT and HIF-2α protein expression. 786-O cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.) or Cul2-targeting siRNA (siRNA Cul2#1). Cell lysates were blotted with anti-CUL2, anti-ARNT, and anti-HIF-2α to identify CUL2, ARNT, and HIF-2α. Anti-β-actin and anti-GAPDH were used as negative controls. B, Cul2 siRNA suppressed ARNT and VEGF mRNA expression. 786-O cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.), Cul2-targeting siRNA (siRNA Cul2#1), or ARNT-targeting siRNA (siRNA ARNT) as indicated. The cells were incubated for 72 h before cell harvest. Results of real-time PCR are presented as fold activation of ARNT and VEGF mRNA normalized to 18 S rRNA. C, ectopically expressed ARNT did not induce HIF-2α protein. 786-O cells were transfected with 100 pmol of siRNA negative control (siRNA Cont.) or Cul2-targeting siRNA (siRNA Cul2#1). One day after siRNA transfection, FLAG hARNT vector or FLAG empty vector was transfected as indicated. The cells were incubated for 72 h after siRNA transfection and 48 h after FLAG expression vector transfection. Cell lysates were blotted with anti-FLAG, anti-CUL2, and anti-HIF-2α to identify FLAG-ARNT, CUL2, and HIF-2α. Anti-β-actin was used as a negative control. D, ectopically expressed ARNT did not reverse the expression of VEGF mRNA suppressed by Cul2 siRNA. 786-O cells were transfected with indicated siRNAs or FLAG hARNT as described in C. The cells were incubated for 72 h before cell harvest. Results of real-time PCR are presented as fold activation of ARNT and VEGF mRNA normalized to 18 S rRNA.
Endogenous CUL2 Is Localized in the Nucleus—To determine the subcellular localization of CUL2, endogenous CUL2 was detected in the nucleus in HeLa cervical cells, H441 lung cells, and 786-O VHL-defective renal cells by immunocytochemistry. In contrast, endogenous VHL was detected in the cytoplasm of HeLa and H441 cells (Fig. 5). In previous studies, exogenously added HA-tagged CUL2 was localized to the cytoplasm and GFP-tagged VHL was localized to the nucleus of COS-7 kidney cells. The HA-tagged CUL2 was translocated into the nucleus after transfection with the GFP-tagged VHL (5). In oocytes of C. elegans, endogenous CUL2 was detected previously in the nucleus (17). This discrepancy may be related to differences between endogenous and exogenous CUL2 or to differences in cell types. Our results indicate that endogenous CUL2 is present in the nucleus, and that its localization is not dependent on VHL, consistent with the concept that nuclear CUL2 influences HIFα activity independently of VHL function in the cytoplasm.
FIGURE 5.
CUL2 is localized in the nucleus, and VHL is localized in the cytoplasm. Immunocytochemistry was performed using HeLa cells (upper panel), H441 cells (middle panel), and 786-O cells (lower panel) with anti-CUL2 and anti-VHL antibodies as described under “Experimental Procedures.” The left panels are merged images.
Cul2 Is Involved in Zebrafish Vascular Development—Because CUL2 was required for HIFα-mediated activation of the VEGF promoter in vitro, we sought to determine whether Cul2 influences vasculogenesis in vivo. zCul2 targeting morpholinos were injected into zebrafish embryos carrying a Flk promoter-driven GFP construct (Flk-GFP) (28). Flk is a tyrosine kinase receptor expressed in endothelial and other cells, and Flk-GFP is selectively expressed in endothelial cells in zebrafish. In vitro, HIF-2α activated the Flk promoter as observed in the VEGF promoter, and its activity was suppressed by Cul2 siRNA (Fig. 6A), indicating that CUL2 influences not only VEGF but also other HIF-mediated transcriptional targets, including Flk.
FIGURE 6.

CUL2 is required for zebrafish (Danio rerio) embryonic development and vasculogenesis. A, Cul2 siRNA suppressed HIF-2α activity of the Flk promoter. HeLa cells were transfected with 1 μg of the hFlk luciferase construct (hFlk-Luciferase), 1 μg of pCMV.β-galactosidase, 1 μg of HIF-2α expression vector (pcDNA3.1.HIF-2α), 100 pmol of siRNA negative control (siRNA Cont.), or Cul2-targeting siRNA (siRNA Cul2#1) as indicated. Results are presented as fold activation of light units normalized to β-galactosidase activity relative to control constructs. B, zCul2 morpholino suppressed CUL2 and VEGF protein expression. Pools of zebrafish embryos (TL) at 48 hpf either injected with zebrafish Cul2-targeting morpholino (zCUL2 MO; 1.15 ng or 2.3 ng per embryo) or non-injected embryos (Control, WT) were collected. The embryo lysates were blotted with anti-CUL2 and anti-zebrafish VEGF to identify CUL2 and VEGF. Anti-β-actin and anti-GAPDH were used as negative controls. C, intersegmental vessels were absent in zCul2 morpholino-injected embryos. Flk-GFP zebrafish embryos injected with non-targeted morpholino (Control MO), non-injected (WT), or zCUL2 morpholino (zCUL2 MO) were examined at 24 hpf. D, zCUL2 morpholino influenced zebrafish development. Flk-GFP zebrafish embryos injected with concentrations of morpholino, ranging from 2.3 to 6.9 ng per embryo, were examined at 24 hpf, resulting in three classes of phenotypes. E, model of vascular gene regulation by CUL2. CUL2 intermediates including ARNT regulate VEGF and Flk gene expression, which in turn controls vasculogenesis.
In zebrafish, zCul2 morpholinos decreased Cul2 protein in the zebrafish embryos and inhibited the expression of zebrafish VEGF protein and mRNA (Fig. 6B and data not shown). β-Actin and GAPDH were not strongly influenced (Fig. 6B). To determine the morphogenetic effects of CUL2 knock-down in the zebrafish, Flk-GFP embryos injected with zCul2 morpholino (6.9 ng) or non-targeted control morpholino (9.2 ng) were examined at 24 hpf (hours post-fertilization). Fluorescence of the entire vasculature including blood vessels of the trunk and body as well as the endocardium was observed in control and wild-type (non-injected) embryos. In contrast, markedly decreased Flk-GFP fluorescence was observed throughout the embryos injected with the zCul2 morpholino (Fig. 6C, middle panels). Fluorescence in the intersegmental vessels was essentially absent (Fig. 6C, upper panels), a phenotype similar to that caused by inhibition of VEGF-A expression with zebrafish VEGF-A morpholinos (36). To assess whether this finding was consistent with the lack of intersegmental vessel formation, embryos were examined at 49 hpf, by which time control embryos have vigorous blood flow visible throughout the embryos. Blood flow through the dorsal aorta, but not through intersegmental vessels, was observed in zCul2 morpholino-injected embryos at this stage (data not shown). Taken together, these data suggest that Cul2 is required for formation of the intersegmental vessels in zebrafish, a finding consistent with the known role of VEGF-A in zebrafish.
Because previous reports suggest that Cul2 may play a role in cell proliferation (17), we wanted to determine whether injection of zebrafish morpholinos creates a novel global defect in morphogenesis. Embryos were injected with increasing concentrations of zCul2 morpholino (2.3-6.9 ng) per embryo. Three classes of phenotypes were observed at 24 hpf (Fig. 6D). Generalized developmental retardation and abnormal intersegmental vessel formation, without other obvious malformations, were observed in the most mildly affected embryos (Class I). Class II defects included moderate developmental retardation with abnormal somite development and corresponding defects in movement (Class II). Severe developmental retardation was apparent in all tissues in the most severely affected embryos (Class III). The severity of the phenotypes seen was related to the amount of morpholino injected, with higher amounts causing more Class III phenotypes (Fig. 6D, table). Thus, at higher concentrations of zCul2 morpholinos, loss of Cul2 caused defects in embryonic growth and patterning. Taken together, Cul2 is required for normal embryonic development and vasculogenesis in zebrafish.
DISCUSSION
Cul2 was previously identified as a component of the VHL tumor suppressor complex that degrades the HIFα protein. The absence of CUL2 stabilized HIFα (Ref. 12 and Fig. 1). Whereas the role of CUL2 on the destabilization of HIFα is well understood, the present study provides support for the novel role of CUL2 required for the activity of HIFα in the expression of VEGF and Flk. In the present study, CUL2 was required for normal development and vasculogenesis in the zebrafish embryo. In vitro, CUL2 was required for HIF activity of both VEGF and Flk transcription, effects that are opposite to that of VHL. Taken together, these findings demonstrate that CUL2 influences embryonic development by at least in part regulating expression of HIF targets such as VEGF and Flk.
In H441 cells, CUL2 influenced HIF-mediated gene expression at least in part by regulating ARNT expression. Whereas both ARNT protein and mRNA were decreased by inhibition of CUL2, the mechanism by which CUL2 regulates ARNT expression remains unknown. CUL2, by associating with ubiquitin ligase, facilitates the degradation of substrate proteins. HIFα is the most common substrate for the CUL2-VHL complex, but in C. elegans, CUL2 degrades TRA-1, the sole member of the Gli family of transcription factors in C. elegans. CUL2 degrades TRA-1 by interacting with FEM-1, a homolog of mammalian FEM1B (12, 37). CUL2 also associates with MED8, a subunit of the transcriptional Mediator complex required for RNA polymerase II function (13), suggesting that CUL2 may degrade other components of the transcriptional regulatory complex to control transcription. In contrast to the finding in H441 cells, ARNT did not reverse the inhibitory effect of Cul2 siRNA in 786-O cells, indicating the existence of CUL2 intermediates/transcriptional factors regulating the HIF-mediated gene expression independently of ARNT. Identification of CUL2 partners or intermediates will help to understand the mechanism of ARNT-dependent or independent gene regulation by CUL2. ARNT has been considered to be a constitutively stable protein. Recently, findings suggested that the levels of ARNT expression were not constitutive but were altered in diseases such as diabetes and lung cancer (38, 39). These findings indicate that the regulation of ARNT by CUL2 may be involved in development and human diseases.
To our knowledge, mouse models useful for understanding the biological functions of CUL2 have not been produced. In C. elegans, Cul2 is expressed in proliferating cells and required for the cell cycle of the G1/S transition and mitosis (17). In the present study, Cul2 was required for the general development of zebrafish embryos, indicating that Cul2 may be involved in cell proliferation or survival, consistent with the finding from the C. elegans study. Cul2 was also required for vasculogenesis, especially formation of the intersegmental vessels in zebrafish. In zebrafish, VEGF-A is known to be required for the formation of the intersegmental vessels (36). The similarity of phenotypes caused by inhibition of both CUL2 and VEGF-A suggests that CUL2 controls intersegmental vessel formation through the regulation of VEGF genes. CUL2 also influenced the activity of the Flk promoter, another known target of HIFα. Interestingly, CUL7, the other member of CULLIN family interacting with RBX1 and SKP1, is also required for vasculogenesis in mice (40), consistent with our findings regarding the role of CUL2 in vasculogenesis in zebrafish. Taken together, these results indicate that Cul2 regulates the expression of VEGF and Flk which in turn influences vasculogenesis.
In the present study, CUL2 induced the degradation of HIFα but simultaneously enhanced the activity of HIFα, indicating two distinct and potentially opposing activities of CUL2. Simultaneous or shared activities mediating both degradation and activation of transcription factors have been proposed as a mechanism to precisely limit transcription (41, 42). For example, SKP2, a component of the CUL1-RING-ubiquitin ligase complex, is oncogenic and overexpressed in human cancers (43, 44). SKP2 ubiquitinates and enhances degradation of the c-Myc protein but also acts as a co-activator (45, 46). The transcriptional activity of HIFα may also be directly associated with its destabilization via CUL2, initiating its degradation.
In summary, CUL2 is known to be a component of the VHL ubiquitination/degradation complex, serving to enhance the degradation of HIFα. Consistent with that concept, suppression of CUL2 expression with Cul2 siRNA increased HIFα protein expression. However, despite the increased levels of the HIFα protein, HIFα-dependent VEGF transcription was concomitantly reduced by Cul2 siRNA, suggesting that CUL2 plays an additional role in the regulation of transcription. In the present study, CUL2, stabilized by RBX1, influenced the expression of ARNT and subsequently HIF-sensitive genes, and in turn embryonic vascular development in zebrafish. Taken together, CUL2 is required for HIF-mediated gene expression and vasculogenesis.
Acknowledgments
We thank R. K. Bruick for providing the HIF-2α expression construct, O. Hankinson for providing the ARNT expression vector, D. Mukhopadhyay for providing the VEGF promoter, C. Patterson for providing the Flk promoter, D. Stainier for providing the Flk1:egfp transgenic zebrafish (Flk-GFP), and J. Shannon and J. Bridges for helpful comments and discussion.
This work was supported, in whole or in part, by National Institutes of Health Grant HL90156 (to J. A. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CUL2, CULLIN 2; VHL, Von Hippel-Lindau; HIF, hypoxia-inducible factor; VEGF, vascular endothelial growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PBS, phosphate-buffered saline; GFP, green fluorescent protein; hpf, hours post-fertilization; DFO, deferoxamine mesylate; RT, reverse transcriptase.
References
- 1.Petroski, M. D., and Deshaies, R. J. (2005) Nat. Rev. Mol. Cell Biol. 6 9-20 [DOI] [PubMed] [Google Scholar]
- 2.Kamura, T., Koepp, D. M., Conrad, M. N., Skowyra, D., Moreland, R. J., Iliopoulos, O., Lane, W. S., Kaelin, W. G., Jr., Elledge, S. J., Conaway, R. C., Harper, J. W., and Conaway, J. W. (1999) Science 284 657-661 [DOI] [PubMed] [Google Scholar]
- 3.Lisztwan, J., Imbert, G., Wirbelauer, C., Gstaiger, M., and Krek, W. (1999) Genes Dev. 13 1822-1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonergan, K. M., Iliopoulos, O., Ohh, M., Kamura, T., Conaway, R. C., Conaway, J. W., and Kaelin, W. G., Jr. (1998) Mol. Cell Biol. 18 732-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pause, A., Lee, S., Worrell, R. A., Chen, D. Y., Burgess, W. H., Linehan, W. M., and Klausner, R. D. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 2156-2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockman, M. E., Masson, N., Mole, D. R., Jaakkola, P., Chang, G. W., Clifford, S. C., Maher, E. R., Pugh, C. W., Ratcliffe, P. J., and Maxwell, P. H. (2000) J. Biol. Chem. 275 25733-25741 [DOI] [PubMed] [Google Scholar]
- 7.Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J. M., Lane, W. S., and Kaelin, W. G., Jr. (2001) Science 292 464-468 [DOI] [PubMed] [Google Scholar]
- 8.Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., Kriegsheim, A. V., Hebestreit, H. F., Mukherji, M., Schofield, C. J., Maxwell, P. H., Pugh, C. W., and Ratcliffe, P. J. (2001) Science 292 468-472 [DOI] [PubMed] [Google Scholar]
- 9.Kamura, T., Sato, S., Iwai, K., Czyzyk-Krzeska, M., Conaway, R. C., and Conaway, J. W. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 10430-10435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohh, M., Park, C. W., Ivan, M., Hoffman, M. A., Kim, T. Y., Huang, L. E., Pavletich, N., Chau, V., and Kaelin, W. G., Jr. (2000) Nat. Cell Biol. 2 423-427 [DOI] [PubMed] [Google Scholar]
- 11.Tanimoto, K., Makino, Y., Pereira, T., and Poellinger, L. (2000) EMBO J. 19 4298-4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamura, T., Maenaka, K., Kotoshiba, S., Matsumoto, M., Kohda, D., Conaway, R. C., Conaway, J. W., and Nakayama, K. I. (2004) Genes Dev. 18 3055-3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brower, C. S., Sato, S., Tomomori-Sato, C., Kamura, T., Pause, A., Stearman, R., Klausner, R. D., Malik, S., Lane, W. S., Sorokina, I., Roeder, R. G., Conaway, J. W., and Conaway, R. C. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 10353-10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clifford, S. C., Walsh, S., Hewson, K., Green, E. K., Brinke, A., Green, P. M., Gianelli, F., Eng, C., and Maher, E. R. (1999) Genes Chromosomes Cancer 26 20-28 [PubMed] [Google Scholar]
- 15.Clifford, S. C., Astuti, D., Hooper, L., Maxwell, P. H., Ratcliffe, P. J., and Maher, E. R. (2001) Oncogene 20 5067-5074 [DOI] [PubMed] [Google Scholar]
- 16.Duerr, E. M., Gimm, O., Neuberg, D. S., Kum, J. B., Clifford, S. C., Toledo, S. P., Maher, E. R., Dahia, P. L., and Eng, C. (1999) J. Clin. Endocrinol. Metab. 84 3207-3211 [DOI] [PubMed] [Google Scholar]
- 17.Feng H., Zhong, W., Punkosdy, G., Gu, S., Zhou, L., Seabolt, E. K., and Kipreos, E. T. (1999) Nat. Cell Biol. 1 486-492 [DOI] [PubMed] [Google Scholar]
- 18.Wenger, R. H., Stiehl, D. P., and Camenisch, G. (2005) Sci. STKE 306 re12. [DOI] [PubMed] [Google Scholar]
- 19.Park, S. K., Dadak, A. M., Haase, V. H., Fontana, L., Giaccia, A. J., and Johnson, R. S. (2003) Mol. Cell Biol. 23 4959-4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida, T., Rossignol, F., Matthay, M. A., Mounier, R., Couette, S., Clottes, E., and Clerici, C. (2004) J. Biol. Chem. 279 14871-14878 [DOI] [PubMed] [Google Scholar]
- 21.Wagner, K. F., Hellberg, A. K., Balenger, S., Depping, R., Dodd-O, J., Johns, R. A., and Li, D. (2004) Am. J. Respir. Cell Mol. Biol. 31 276-282 [DOI] [PubMed] [Google Scholar]
- 22.Tian, H., McKnight, S. L., and Russell, D. W. (1997) Genes Dev. 11 72-82 [DOI] [PubMed] [Google Scholar]
- 23.Hoffman, E. C., Reyes, H., Chu, F. F., Sander, F., Conley, L. H., Brooks, B. A., and Hankinson, O. (1991) Science 252 954-958 [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay, D., Knebelmann, B., Cohen, H. T., Ananth, S., and Sukhatme, V. P. (1997) Mol. Cell Biol. 17 5629-5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson, C., Perrella, M. A., Hsieh, C. M., Yoshizumi, M., Lee, M. E., and Haber, E. (1995) J. Biol. Chem. 270 23111-23118 [DOI] [PubMed] [Google Scholar]
- 26.Maeda, Y., Hunter, T. C., Loudy, D. E., Dave, V., Schreiber, V., and Whitsett, J. A. (2006) J. Biol. Chem. 281 9600-9606 [DOI] [PubMed] [Google Scholar]
- 27.Carter, C., Pan, S., Zouhar, J., Avila, E. L., Girke, T., and Raikhel, N. V. (2004) Plant Cell 16 3285-3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, S. W., Beis, D., Mitchell, T., Chen, J. N., and Stainier, D. Y. (2005) Development 132 5199-5209 [DOI] [PubMed] [Google Scholar]
- 29.Warnecke, C., Zaborowska, Z., Kurreck, J., Erdmann, V. A., Frei, U., Wiesener, M., and Eckardt, K. U. (2004) FASEB J. 18 1462-1464 [DOI] [PubMed] [Google Scholar]
- 30.Olenyuk, B. Z., Zhang, G. J., Klco, J. M., Nickols, N. G., Kaelin, W. G., Jr., and Dervan, P. B. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 16768-16773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Rourke, J. F., Tian, Y. M., Ratcliffe, P. J., and Pugh, C. W. (1999) J. Biol. Chem. 274 2060-2071 [DOI] [PubMed] [Google Scholar]
- 32.Feng, Y., Lee, N., and Fearon, E. R. (2003) Cancer Res. 63 8726-8734 [PubMed] [Google Scholar]
- 33.Wood, M. A., McMahon, S. B., and Cole, M. D. (2000) Mol. Cell 5 321-330 [DOI] [PubMed] [Google Scholar]
- 34.Ikura, T., Ogryzko, V. V., Grigoriev, M., Groisman, R., Wang, J., Horikoshi, M., Scully, R., Qin, J., and Nakatani, Y. (2000) Cell 102 463-473 [DOI] [PubMed] [Google Scholar]
- 35.Maxwell, P. H., Wiesener, M. S., Chang, G. W., Clifford, S. C., Vaux, E. C., Cockman, M. E., Wykoff, C. C., Pugh, C. W., Maher, E. R., and Ratcliffe, P. J. (1999) Nature 399 271-275 [DOI] [PubMed] [Google Scholar]
- 36.Nasevicius, A., Larson, J., and Ekker, S. C. (2000) Yeast 17 294-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starostina, N. G., Lim, J. M., Schvarzstein, M., Wells, L., Spence, A. M., and Kipreos, E. T. (2007) Dev. Cell 13 127-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunton, J. E., Kulkarni, R. N., Yim, S., Okada, T., Hawthorne, W. J., Tseng, Y. H., Roberson, R. S., Ricordi, C., O'Connell, P. J., Gonzalez, F. J., and Kahn, C. R. (2005) Cell 122 337-349 [DOI] [PubMed] [Google Scholar]
- 39.Weir, B. A., Woo, M. S., Getz, G., Perner, S., Ding, L., Beroukhim, R., Lin, W. M., Province, M. A., Kraja, A., Johnson, L. A., Shah, K., Sato, M., Thomas, R. K., Barletta, J. A., Borecki, I. B., Broderick, S., Chang, A. C., Chiang, D. Y., Chirieac, L. R., Cho, J., Fujii, Y., Gazdar, A. F., Giordano, T., Greulich, H., Hanna, M., Johnson, B. E., Kris, M. G., Lash, A., Lin, L., Lindeman, N., Mardis, E. R., McPherson, J. D., Minna, J. D., Morgan, M. B., Nadel, M., Orringer, M. B., Osborne, J. R., Ozenberger, B., Ramos, A. H., Robinson, J., Roth, J. A., Rusch, V., Sasaki, H., Shepherd, F., Sougnez, C., Spitz, M. R., Tsao, M. S., Twomey, D., Verhaak, R. G., Weinstock, G. M., Wheeler, D. A., Winckler, W., Yoshizawa, A., Yu, S., Zakowski, M. F., Zhang, Q., Beer, D. G., Wistuba, I. I., Watson, M. A., Garraway, L. A., Ladanyi, M., Travis, W. D., Pao, W., Rubin, M. A., Gabriel, S. B., Gibbs, R. A., Varmus, H. E., Wilson, R. K., Lander, E. S., and Meyerson, M. (2007) Nature 450 893-89817982442 [Google Scholar]
- 40.Arai, T., Kasper, J. S., Skaar, J. R., Ali, S. H., Takahashi, C., and DeCaprio, J. A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 9855-9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodadek, T., Sikder, D., and Nalley, K. (2006) Cell 127 261-264 [DOI] [PubMed] [Google Scholar]
- 42.Muratani, M., and Tansey, W. P. (2003) Nat. Rev. Mol. Cell Biol. 4 192-201 [DOI] [PubMed] [Google Scholar]
- 43.Gstaiger, M., Jordan, R., Lim, M., Catzavelos, C., Mestan, J., Slingerland, J., and Krek, W. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 5043-5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng, N., Schulman, B. A., Song, L., Miller, J. J., Jeffrey, P. D., Wang, P., Chu, C., Koepp, D. M., Elledge, S. J., Pagano, M., Conaway, R. C., Conaway, J. W., Harper, J. W., and Pavletich, N. P. (2002) Nature 416 703-709 [DOI] [PubMed] [Google Scholar]
- 45.Kim, S. Y., Herbst, A., Tworkowski, K. A., Salghetti, S. E., and Tansey, W. P. (2003) Mol. Cell 11 1177-1188 [DOI] [PubMed] [Google Scholar]
- 46.von der Lehr, N., Johansson, S., Wu, S., F. Bahram, F., Castell, A., Cetinkaya, C., Hydbring, P., Weidung, I., Nakayama, K., Nakayama, K. I., Soderberg, O., Kerppola, T. K., and Larsson, L. G. (2003) Mol. Cell 11 1189-1200 [DOI] [PubMed] [Google Scholar]