Ch2 is a major lipid component in eukaryotic cells and can attain levels as high as 50% (on a molar basis) of all lipids present in the plasma membrane of certain cells (1). The pathways responsible for Ch accumulation at this site are a consequence of gene regulation largely worked out by Brown and Goldstein (2). The very high concentration of this unique and singular lipid at the plasma membrane of cells places it in a position to encounter ROS that cross this permeability barrier. Ch can be oxidized in membranes even when polyunsaturated fatty acyl groups are not. Also, some unique products can serve as markers of certain ROS.
The structure of Ch makes it susceptible to a variety of radical as well as nonradical oxidation reactions due to the 5,6-double bond and the concomitant vinylic methylene group at C-7 in the B ring. The isooctyl side chain at C-17 is a site for enzymatic oxidation largely by P450 cytochromes to form a series of biochemically active oxysterols, but this site of oxidation is typically not a target for ROS relevant to cellular biochemistry (3). Oxysterols are clearly formed in cells, but considerable evidence exists for a dietary origin of some oxysterols transported to cells by chylomicrons (4). Various mechanisms, including heating of foods in air, can lead to formation of oxysterols that can be ingested. Several excellent reviews by Smith (3, 5) have covered this area, and although somewhat older literature, they are a wealth of information about Ch autoxidation.
Free Radical Pathways
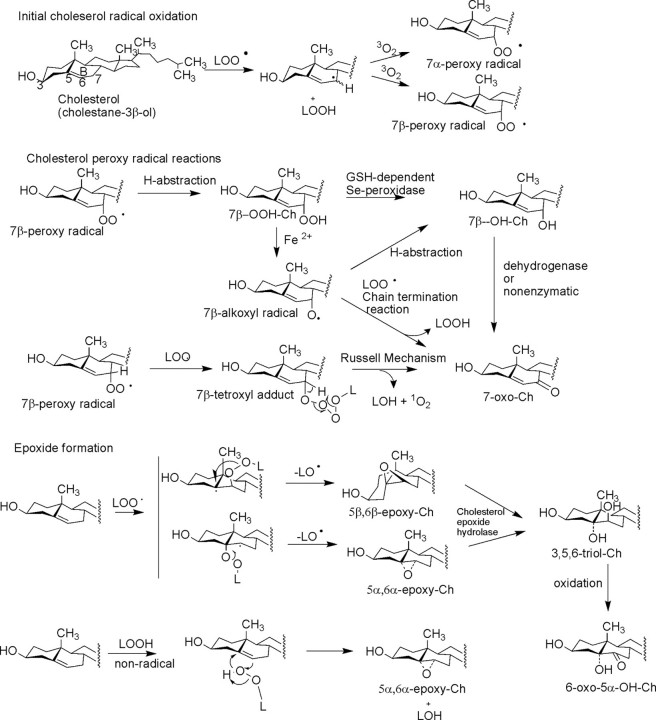
The free radical-based oxidation of Ch has been the focus of several biochemical studies (6). The fairly complex mixture of oxysterols that result from free radical-mediated Ch oxidation confounds insight into the exact mechanism of formation of individual oxysterols. This is due in part to the random character of free radical oxidation as well as subsequent rearrangement of reactive intermediates. Four major products are typically observed, 7α,β-OOH-Ch, 7α,β-OH-Ch, 7-ox-Ch, and 5,6-epoxy-Ch (abbreviations and nomenclature are listed in supplemental Table 1), but many minor products, including A ring-oxidized species, are known (3, 5). The generation of highly reactive hydroxyl radicals (OH•) by various mechanisms, including H2O2 reduction by superoxide anion catalyzed by iron(II) (Fenton reaction), peroxynitrite (ONOO–) following the reaction of nitric oxide with superoxide anion, or ionizing radiation, can all eventually lead to abstraction of a C-7 allylic hydrogen atom, forming a carbon-centered radical at this site. The focus of these free radical oxidations of Ch is at C-7 because the carbon–hydrogen bond is sufficiently weak at this position (bond dissociation energy of 88 kcal/mol (7)) that longer lived lipid peroxy radical or alkoxyl radicals derived from polyunsaturated fatty acyl lipid peroxyl radicals (LOO•) can readily abstract this hydrogen atom. The C-7-centered radical intermediate of Ch has a sufficiently long half-life to encounter a ground state molecular oxygen molecule, forming a hydroperoxy radical. This means that the propagation of free radical chemistry through the lipid bilayer, which might be initiated at some point by hydroxyl radical, can lead to the observed peroxidation of Ch. This hydroperoxy radical intermediate can abstract certain hydrogen atoms and thus propagate the free radical reaction, forming (after hydrogen abstraction) chemically stable 7α- and 7β-OOH-Ch (Fig. 1). Interestingly, recent evidence has shown that Ch hydroperoxides move much more efficiently between various cellular compartments compared with Ch itself (8) and thus become available for many sites within the cell for further reactions, including reductions by glutathione-dependent selenoperoxidases (6) or by nonenzymatic reactions with chelated transition metals (7). Reduction of 7α,β-OOH-Ch by iron(II) can lead to a 7α,β-alkoxyl-Ch radical that can undergo several different pathways of reaction. For example, hydrogen abstraction from another molecule results in continued free radical propagation and formation of stable 7α,β-OH-Ch.
FIGURE 1.
Pathways of free radical oxidation of Ch by initial abstraction of a C-7 hydrogen atom and subsequent reactions of peroxy radicals. 5,6-Epoxy-Ch can be made by both radical and nonradical mechanisms. LOO• refers to a lipid hydroperoxy radical intermediate; LOH refers to a hydroxy lipid; LOOH refers to a lipid hydroperoxide; and LO• refers to a lipid alkoxyl radical intermediate.
The formation of the 7-oxo-Ch product is thought to be due in part to the reaction of this 7α,β-alkoxyl radical with another lipid peroxy radical, which abstracts the remaining proton at C-7 and forms the 7-oxo product in a radical propagation termination reaction (9). Another mechanism for the formation of the typically abundant 7-oxo-Ch can derive from interaction of two hydroperoxy radicals in a “Russell mechanism” (10) and a cyclic tetroxide intermediate (Fig. 1). The products of this reaction would be 7-oxo-Ch, a hydroxy lipid, and singlet oxygen (1O2) to conserve electronic spin states in this mechanism (10). 7-Oxo-Ch can also result from enzymatic oxidation of 7-OH-Ch (11). These multiple pathways may help to explain the abundance of this product.
Radical hydrogen atom abstraction at a bisallylic methylene group found in polyunsaturated fatty acyl moieties of phospholipids would be expected to be energetically more favorable relative to the C-7 methylene group in Ch. Indeed, in plasma, the formation of oxidized polyunsaturated fatty acyl products was observed to be 30-fold higher than that of oxidized Ch (12), yet in a lipid bilayer, the structured environment (entropy) can alter the propagation path of free radical events, likely as a result of the location of radicals with respect to ordered Ch. Studies have been carried out in cells in which it was observed that phospholipids containing polyunsaturated fatty acids were less susceptible to oxidation (13); however, plasmalogen phospholipids were found to inhibit Ch peroxidation (14). These studies have revealed extensive oxidation of Ch prior to oxidation at the bisallylic methylene groups of the phospholipids. The propagation of radical events through a lipid bilayer is likely the mechanism by which Ch eventually becomes oxidized at C-7 due to the high abundance of this single lipid.
Other products of Ch radical oxidation are the epimeric 5,6-epoxides (Fig. 1). Both 5α,6α- and 5β,6β-epoxy-Ch are observed as products. Interestingly, these products require an intermediate formation of hydroperoxy lipids and are not the primary products of oxidation at C-7 of Ch (3). The formation of these epoxy-Ch metabolites can be a result of a radical-based mechanism of attack of LOO• at the Δ5,6-double bond, forming an initial radical adduct at C-5 or C-6 (Fig. 1) followed by loss of an alkoxyl lipid radical (15). A nonradical-based mechanism for the formation of these epoxy-Ch has been proposed based upon the known epoxidation of olefins by hydroperoxides (3, 4). These 5,6-epoxides are observed as the most abundant nonenzymatically formed oxysterols in the macrophage.3
This nonradical mechanism has been suggested to account for the occurrence of epoxy-Ch derived from Ch esters in oxidized LDL. In this case, the initial peroxidation site is at the fatty acyl moiety such as the linoleoyl acyl group and initial formation of a linoleoyl hydroperoxide. This hydroperoxide at C-9 or C-13 of the fatty acyl chain could rearrange, intramolecularly, to the corresponding epoxy-Ch with the hydroperoxy group reduced to a hydroxy moiety (16).
Nonradical Pathways (1O2, O3, HOCl)
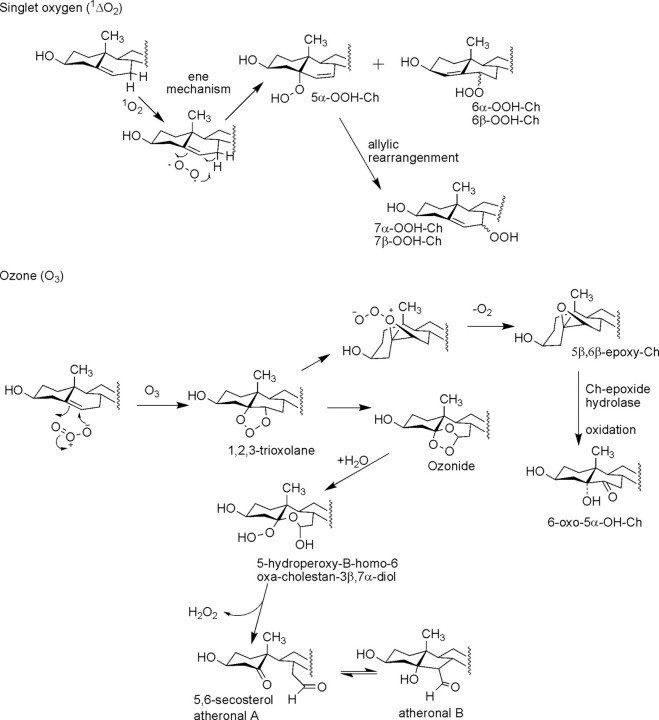
Several nonradical pathways of Ch peroxidation can occur in cellular membranes that are not dependent upon oxygen- or carbon-centered radicals that do not result in free radical propagation reactions through phospholipids, at least initially. One widely studied reaction has been that with singlet oxygen (1ΔgO2, abbreviated 1O2 is on the order of a few microseconds, this reactive form of oxygen can travel only a fraction of the cell diameter of a typical eukaryotic cell, yet singlet oxygen readily produces one major and two minor products by an “ene” reaction mechanism with Ch (Fig. 2).
FIGURE 2.
Pathways of nonradical modification of Ch by singlet oxygen and ozone.
The reaction of singlet oxygen to form 5α-OOH-Ch has been found to have the highest rate constant relative to formation of 6α,β-OOH-Ch. Furthermore, the 5α-OOH-Ch product is metabolized at a lower rate, which helps explain the accumulation of this oxidized metabolite of Ch when singlet oxygen is exposed to cells (18). The initially formed 5α-OOH-Ch is also known to rearrange to 7α,β-OOH-Ch upon sample work-up and possibly in situ in the membrane (6, 19). These observations have led to the suggestion that measurement of 5α-OOH-Ch could be a marker for singlet oxygen production within cells (17).
The reaction of Ch with ozone has been known for some time to be both rapid and complex (3). The lung is particularly susceptible to ozone due to the presence of ozone in the troposphere and therefore present in air inspired into the lung. Recent studies have revealed the formation of two major products when low concentrations (100–500 ppb) of ozone (such levels can be present in the air of large cities, to which humans are exposed on a constant basis) were exposed to lung surfactant (20). The initial reaction of ozone is the addition across the Δ5-double bond of Ch, which yields a 1,2,3-trioxolane that undergoes rapid and spontaneous decomposition to yield several additional reactive entities, including carbonyl oxides, carbon-centered radicals, and hydroperoxides, which ultimately lead to the cleavage of the C-5–C-6 double bond (Fig. 2). Some of the more stable ozonide products have been recently structurally characterized (21). Peroxidation of polyunsaturated fatty acyl groups is also initiated by reaction of ozone, leading to the formation of abundant phospholipid hydroperoxides. The second major product of Ch when ozone was exposed to surfactant was identified as 5,6β-epoxy-Ch, which could be formed by rearrangement of the 1,2,3-trioxolane intermediate (Fig. 2) or by a phospholipid or Ch hydroperoxide mechanism as described above (Fig. 1).
Recently, a set of Ch ozonolysis products was described as being formed in atherosclerotic plaques as a result of in situ formation of ozone (22). Although there is some controversy about the identification of the products and mechanism of formation of ozone by this model (23), nonetheless, these products do possess potent activities on cells. These metabolites, atheronals A and B (Fig. 2), would result from the expected cleavage of the B ring and aldol condensation (22, 24).
The abundant production of HOCl by neutrophils and HOBr by eosinophils leads to the potential exposure of these very ROS to Ch during phagocytosis. Hypochlorous acid is known to react with olefins to form chlorohydrins by simple electrophilic addition across the double bond. Such oxidation products have been found following reaction with HOCl (25). Unexpectedly, there was evidence for the addition of two chlorine atoms across the 5,6-double bond of Ch from Cl2, yielding a very nonpolar 5,6-dichlorocholestane metabolite. When fatty streaks from atherosclerotic plaques were examined, this metabolite was readily detected (26).
Nonenzymatically Formed Oxysterols in Pathophysiology
The recognition that Ch and Ch esters can be oxidized by nonenzymatic means has driven two very separate types of studies: (i) to assess the involvement of ROS in a disease process and to identify marker products and (ii) to assess the role of oxysterols in a disease process by way of mediating biological responses. The first type of study demands specific and sensitive analytical capability, which has substantially improved in the past decade with the development of electrospray ionization mass spectrometry and liquid chromatography/tandem mass spectrometry. It was not possible to analyze species such as the hydroperoxides of Ch directly by gas chromatography/mass spectrometry. Now even reactive hydroxy, hydroperoxy, and intact ozonides can be directly analyzed using electrospray ionization (21). This capability has changed both types of oxysterol studies because it is now possible to detect those oxysterol intermediates and to focus attention on additional oxysterol entities that could mediate biological activities.
Recent examples include measurement of the specific oxysterols that are formed in both type 1 and type 2 DM in kidney, heart, and liver (27). It was found that levels of 7α- and 7β-OOH-Ch as well as 7α- and 7β-OH-Ch were increased in all tissues analyzed, consistent with initiation of free radical lipid peroxidation. The plasma of patients with DM has been studied, and the oxysterols 7α- and 7β-OH-Ch, and 5α,6α- and 5β,6β-epoxy-Ch were measured. These oxysterols increased in the DM plasma, and these oxysterols have been suggested to be useful as biomarkers for oxidative damage in patients with DM (28).
Studies probing the biological action of oxysterols had been an active area of investigation, and many aspects have been reviewed extensively (29–31). Recent studies have focused considerably on the role oxysterols play as mediators in atherosclerosis, cytotoxicity, and regulation of Ch biosynthesis.
Atherosclerosis
Many studies have now established that various oxysterols can be found in fatty streaks, aortic plaques, and even aortic tissue of human atherosclerotic plaques (32). The post-mortem analysis of aortic tissue from rabbits fed a high Ch diet revealed the presence of several oxysterols at increased levels in atherosclerotic areas (33). In human studies, 7α- and 7β-OH-Ch, 5,6-epoxy-Ch, and 7-oxo-Ch were found at substantially higher levels in fatty streaks and in advanced lesions compared with normal tissues (34).
These observations led to detailed studies of the biological activity of certain oxysterols; however, their involvement in atherosclerosis remains unclear. Early stage atherosclerosis involves conversion of the macrophage to foam cells due in part to the uptake of oxidized LDL. Detailed studies of the lipids present in oxidized LDL have led to identification of a host of different oxidized phospholipids as well as oxysterols. Therefore, probing the activity of specific oxysterols added to endothelial cells has been a logical experiment considering that ROS-derived oxysterols are cytotoxic and may induce apoptosis. However, these pharmacological studies in many cases employed high concentrations of a single oxysterol, which is unlikely to occur in vivo. In fact, when mixtures of oxysterols at relevant concentrations were added to cells, it was difficult to find substantial biological activity (35).
Recently, two specific oxysterols derived from the reaction of ozone with Ch have been proposed to play a role in the early stages of atherosclerosis (22). Pharmacological studies of these products, termed atheronals A and B, with cultured macrophages, human umbilical vein endothelial cells, and human monocytes revealed that both cause chemotaxis and migration of the cells toward the oxysterols (36). Atheronal A can also cause increased expression of the adhesion molecule E-selectin, and atheronal B potentiates differentiation of monocytes into macrophages (36).
Studies of the activities of various oxysterols have led to the description of >10 biological activities relevant to atherosclerosis (37). Additionally, various oxysterols can lead to macrophage cell death in the latter stages of atherosclerosis (38). Finally, 7-oxo-Ch, 5,6-epoxy-Ch, 7-OH-Ch, and cholestanetriol have been shown to increase platelet aggregation, although at relatively high concentrations (39). Although the molecular mechanisms by which these oxysterols exert these activities are often unknown, such studies do present hints as to the potential role that oxysterols, derived from nonenzymatic reactions of Ch with ROS in vivo, may play in a disease as complex as atherosclerosis.
Cytotoxicity of Oxysterols
One of the important toxic effects of nonenzymatically derived oxysterols is the effect on cell viability. Numerous studies have revealed that 7β-OH-Ch, 7-oxo-Ch, and 5β,6β-epoxy-Ch are cytotoxic at relatively low concentrations (25 nm), most likely by inducing cellular apoptosis (40). The possible mechanism for induction of apoptosis in cells like smooth muscle cells may be related to the altered biophysical properties of membranes within the cells such as the ER (31). Because various oxysterols, including 7β-OOH-Ch, 7β-OH-Ch, 7-oxo-Ch, and 5,6-epoxy-Ch, are found in oxidized LDL, pharmacological studies of these agents have been carried out, resulting in the observations that these could mimic the cytotoxic effects of oxidized LDL (41). In addition to the Ch products initially formed by ROS, subsequent enzymatic metabolism can occur. For example, 5β,6β-epoxy-Ch is a substrate for epoxide hydrolase to yield the corresponding 3,5,6-triol-Ch (42). This metabolite has its own activity (43) and can be also metabolized to a unique metabolite that was found in pulmonary epithelial cells as 6-oxo-5-OH-Ch (44). This semi-enzymatically derived oxysterol was found to have considerable biological activity and perhaps accounted for the majority of the activity of 5,6β-epoxy-Ch in epithelial cells in terms of both cytotoxicity and inhibiting Ch biosynthesis.
Detailed studies of the mechanism of cellular apoptosis induced by oxysterols, e.g. 7-oxo-Ch, have been reviewed (45). In general, it is thought that these oxysterols induce cellular apoptosis either through the release of cytochrome c from the mitochondrion and then activation of caspase-9 (mitochondrial pathway) or through the Fas/caspase pathway.
Oxysterols and Regulation of Ch
It has been known for some time that oxysterols are capable of inhibiting Ch biosynthesis (3). Although many of the products of nonenzymatic oxidation of Ch can inhibit 3-hydroxy-3-methylglutaryl-CoA reductase (29), only relatively recently has a more detailed understanding concerning the complexity of this event emerged. It is thought that the inhibition of Ch biosynthesis by itself and oxysterols occurs by way of blocking formation of a peptide fragment cleaved from SREBPs. For the transcriptionally active peptide to be proteolytically cleaved and travel into the nucleus, the SREBPs must bind to another protein called Scap, and this Scap-SREBP complex must leave the ER and be transported to the Golgi apparatus. This transport process involves Scap-SREBP binding to COPII proteins, which concentrates the Scap-SREBP complex into vesicles that bud from the ER membrane and then, through vesicular transport, become fused with the Golgi apparatus (46). Ch inhibits this pathway by inducing Scap-SREBP to bind to two other proteins called Insig-1 and Insig-2, thereby blocking association of the Scap component of the complex with the COPII proteins (47). Once Scap has bound to the Insig proteins, the Scap-SREBP complex cannot move to the Golgi apparatus. Recent evidence has suggested that some oxysterols, primarily the enzymatically formed 25-hydroxy-Ch, bind to the Insig proteins rather than the Scap proteins (47). It is not clear, however, if a role is played by the nonenzymatically formed oxysterols because they were intermediate in potency in binding to either Scap or Insig. An interesting aspect of these detailed studies was the realization that Ch and oxysterols were not altering the biophysical properties of the membrane, but rather exerting their effect through receptor-ligand-like interactions with specific proteins (48, 49). A large number of oxysterols can be generated by the various ROS, but relatively few have ever been tested in binding to either Scap or the Insig proteins. It is possible that some oxidized Ch products from the nonenzymatic pathway could have different effects in particular when considering the more reactive intermediate such as the hydroperoxy and subsequent metabolites generated by metabolic processing of oxysterols within the cell.
Conclusion
The nonenzymatic oxidation of Ch leads to a host of oxysterols, the structures of which are largely dependent upon the ROS that initiate the covalent modification of Ch. When ROS mediate free radical oxidation of Ch, a very diverse and complex mixture of oxysterols results from both the initial oxygenated species of Ch and their subsequent metabolites, which can be formed within the cell. Other ROS, including ozone, are becoming better understood in terms of the complexity of the initial covalently modified Ch intermediates as well as activities of subsequent metabolites. These molecules may exert their activity through a biophysical modification of the membrane on the organization of both lipids and proteins within membranes. It is also possible that these molecules bind to regulatory proteins involved in Ch biosynthesis or perhaps other lipid biochemical pathways. These and other effects may be mediated through a receptor-ligand interaction with either intracellular or extracellular proteins. A detailed understanding of the role that various oxysterols play in disease is still not at hand, largely because of the complexity of the mixture of oxysterols present in vivo, the potential interaction of various pathways, and the observed integrated response of the cell to the oxysterols. Major advances in being able to determine the structures of the covalently modified Ch by ROS have been made recently, which will further expand our understanding of the total number and concentrations of different products and perhaps provide better insight into the role that these molecules play in pathophysiology.
This work was supported, in whole or in part, by National Institutes of Health Grant HL034303. This is the third article of seven in the Oxidized Lipids Minireview Series. This minireview will be reprinted in the 2008 Minireview Compendium, which will be available in January, 2009.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
Footnotes
The abbreviations used are: Ch, cholesterol(s); ROS, reactive oxygen species; LDL, low density lipoprotein; DM, diabetes mellitus; ER, endoplasmic reticulum; SREBP, sterol regulatory element-binding protein.
D. Russell, personal communication.
References
- 1.Koumanov, K. S., Tessier, C., Momchilova, A. B., Rainteau, D., Wolf, C., and Quinn, P. J. (2005) Arch. Biochem. Biophys. 434 150–158 [DOI] [PubMed] [Google Scholar]
- 2.Brown, M. S., and Goldstein, J. L. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 11041–11048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith, L. L. (1981) Cholesterol Autoxidation, Plenum Press, New York
- 4.Vine, D. F., Croft, K. D., Beilin, L. J., and Mamo, J. C. (1997) Lipids 32 887–893 [DOI] [PubMed] [Google Scholar]
- 5.Smith, L. L. (1987) Chem. Phys. Lipids 44 87–125 [DOI] [PubMed] [Google Scholar]
- 6.Girotti, A. W. (1998) J. Lipid Res. 39 1529–1542 [PubMed] [Google Scholar]
- 7.Gardner, H. W. (1989) Free Radic. Biol. Med. 7 65–86 [DOI] [PubMed] [Google Scholar]
- 8.Vila, A., Levchenko, V. V., Korytowski, W., and Girotti, A. W. (2004) Biochemistry 43 12592–12605 [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y. H., Abdalla, D. S. P., and Sevanian, A. (1997) Free Radic. Biol. Med. 23 202–214 [DOI] [PubMed] [Google Scholar]
- 10.Howard, J. A., and Ingold, K. U. (1968) J. Am. Chem. Soc. 90 1056–1058 [Google Scholar]
- 11.Niki, E., Yoshida, Y., Saito, Y., and Noguchi, N. (2005) Biochem. Biophys. Res. Commun. 338 668–676 [DOI] [PubMed] [Google Scholar]
- 12.Yoshida, Y., and Niki, E. (2004) Free Radic. Res. 38 787–794 [DOI] [PubMed] [Google Scholar]
- 13.Saito, Y., Yoshida, Y., and Niki, E. (2007) FEBS Lett. 581 4349–4354 [DOI] [PubMed] [Google Scholar]
- 14.Maeba, R., and Ueta, N. (2003) J. Lipid Res. 44 164–171 [DOI] [PubMed] [Google Scholar]
- 15.Watabe, T., Tsubaki, A., Isobe, M., Ozawa, N., and Hiratsuka, A. (1984) Biochim. Biophys. Acta 795 60–66 [PubMed] [Google Scholar]
- 16.Spiteller, G. (2006) Free Radic. Biol. Med. 41 362–387 [DOI] [PubMed] [Google Scholar]
- 17.Girotti, A. W., and Korytowski, W. (2000) Methods Enzymol. 319 85–100 [DOI] [PubMed] [Google Scholar]
- 18.Korytowski, W., and Girotti, A. W. (1999) Photochem. Photobiol. 70 484–489 [PubMed] [Google Scholar]
- 19.Beckwith, A. L. J., Davies, A. G., Davison, I. G. E., Maccoll, A., and Mruzek, M. H. (1989) J. Chem. Soc. Perkin Trans. I 2 815–824 [Google Scholar]
- 20.Pulfer, M. K., and Murphy, R. C. (2004) J. Biol. Chem. 279 26331–26338 [DOI] [PubMed] [Google Scholar]
- 21.Pulfer, M. K., Harrison, K., and Murphy, R. C. (2004) J. Am. Soc. Mass Spectrom. 15 194–202 [DOI] [PubMed] [Google Scholar]
- 22.Wentworth, P. J., Nieva, J., Takeuchi, C., Galve, R., Wentworth, A. D., Dilley, R. B., DeLaria, G. A., Saven, A., Babior, B. M., Janda, K. D., Eschenmoser, A., and Lerner, R. A. (2003) Science 302 1053–1056 [DOI] [PubMed] [Google Scholar]
- 23.Smith, L. L. (2004) Free Radic. Biol. Med. 37 318–324 [DOI] [PubMed] [Google Scholar]
- 24.Wang, K., Bermudez, E., and Pryor, W. A. (1993) Steroids 58 225–229 [DOI] [PubMed] [Google Scholar]
- 25.van den Berg, J. J., Winterbourn, C. C., and Kuypers, F. A. (1993) J. Lipid Res. 34 2005–2012 [PubMed] [Google Scholar]
- 26.Hazen, S. L., Hsu, F. F., Duffin, K., and Heinecke, J. W. (1996) J. Biol. Chem. 271 23080–23088 [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka, N., Adachi, J., Ueno, Y., and Yoshida, K. (2005) Free Radic. Res. 39 299–304 [DOI] [PubMed] [Google Scholar]
- 28.Ferderbar, S., Pereira, E. C., Apolinario, E., Bertolami, M. C., Faludi, A., Monte, O., Calliari, L. E., Sales, J. E., Gagliardi, A. R., Xavier, H. T., and Abdalla, D. S. (2007) Diabetes Metab. Res. Rev. 23 35–42 [DOI] [PubMed] [Google Scholar]
- 29.Smith, L. L., and Johnson, B. H. (1989) Free Radic. Biol. Med. 7 285–332 [DOI] [PubMed] [Google Scholar]
- 30.Bjorkhem, I., and Diczfalusy, U. (2002) Arterioscler. Thromb. Vasc. Biol. 22 734–742 [DOI] [PubMed] [Google Scholar]
- 31.Massey, J. B. (2006) Curr. Opin. Lipidol. 17 296–301 [DOI] [PubMed] [Google Scholar]
- 32.Brown, A. J., and Jessup, W. (1999) Atherosclerosis 142 1–28 [DOI] [PubMed] [Google Scholar]
- 33.Hodis, H. N., Crawford, D. W., and Sevanian, A. (1991) Atherosclerosis 89 117–126 [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Cruset, S., Carpenter, K. L., Guardiola, F., Stein, B. K., and Mitchinson, M. J. (2001) Free Radic. Res. 35 31–41 [DOI] [PubMed] [Google Scholar]
- 35.Leonarduzzi, G., Biasi, F., Chiarpotto, E., and Poli, G. (2004) Mol. Aspects Med. 25 155–167 [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi, C., Galve, R., Nieva, J., Witter, D. P., Wentworth, A. D., Troseth, R. P., Lerner, R. A., and Wentworth, P., Jr. (2006) Biochemistry 45 7162–7170 [DOI] [PubMed] [Google Scholar]
- 37.Colles, S. M., Maxson, J. M., Carlson, S. G., and Chisolm, G. M. (2001) Trends Cardiovasc. Med. 11 131–138 [DOI] [PubMed] [Google Scholar]
- 38.Maxfield, F. R., and Tabas, I. (2005) Nature 438 612–621 [DOI] [PubMed] [Google Scholar]
- 39.Selley, M. L., McGuiness, J. A., and Ardlie, N. G. (1996) Thromb. Res. 83 449–461 [DOI] [PubMed] [Google Scholar]
- 40.Lemaire-Ewing, S., Prunet, C., Montange, T., Vejux, A., Berthier, A., Bessede, G., Corcos, L., Gambert, P., Neel, D., and Lizard, G. (2005) Cell Biol. Toxicol. 21 97–114 [DOI] [PubMed] [Google Scholar]
- 41.Lizard, G., Lemaire, S., Monier, S., Gueldry, S., Neel, D., and Gambert, P. (1997) FEBS Lett. 419 276–280 [DOI] [PubMed] [Google Scholar]
- 42.Newman, J. W., Morisseau, C., and Hammock, B. D. (2005) Prog. Lipid Res. 44 1–51 [DOI] [PubMed] [Google Scholar]
- 43.Wu, Q., and Huang, K. (2006) Biochim. Biophys. Acta 1761 350–359 [DOI] [PubMed] [Google Scholar]
- 44.Pulfer, M. K., Taube, C., Gelfand, E., and Murphy, R. C. (2005) J. Pharmacol. Exp. Ther. 312 256–264 [DOI] [PubMed] [Google Scholar]
- 45.Panini, S. R., and Sinensky, M. S. (2001) Curr. Opin. Lipidol. 12 529–533 [DOI] [PubMed] [Google Scholar]
- 46.Radhakrishnan, A., Sun, L. P., Kwon, H. J., Brown, M. S., and Goldstein, J. L. (2004) Mol. Cell 15 259–268 [DOI] [PubMed] [Google Scholar]
- 47.Sun, L. P., Seemann, J., Goldstein, J. L., and Brown, M. S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 6519–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radhakrishnan, A., Ikeda, Y., Kwon, H. J., Brown, M. S., and Goldstein, J. L. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 6511–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Infante, R. E., Abi-Mosleh, L., Radhakrishnan, A., Dale, J. D., Brown, M. S., and Goldstein, J. L. (2008) J. Biol. Chem. 283 1052–1063 [DOI] [PubMed] [Google Scholar]