Homolytic decomposition of PUFA2-derived lipid hydroperoxides results in formation of the α,β-unsaturated aldehydic bifunctional electrophiles DDE, EDE, HNE, HPNE, MDA (shown as β-hydroxyacrolein), ONE, DODE, and 5,8-dioxo-(10E)-octenoic acid (see Fig. 1) (1–3). Intracellular formation of the bifunctional electrophiles can then result in the formation of GSH, protein, and DNA adducts (1–3, 5). The analysis of lipid hydroperoxide-derived DNA adducts can facilitate molecular epidemiology studies by providing insight into the amount of a genotoxin that has reached the DNA of the tissue under study (6, 7). DNA repair enzymes, such as those involved in base excision repair, are able to excise the DNA adducts so that they can potentially be excreted in the urine (7). This suggests that non-invasive MS-based techniques could be used to monitor urinary DNA adducts arising from lipid hydroperoxide-mediated DNA damage. Unfortunately, to date, the analysis of urinary DNA adducts of lipid hydroperoxide-derived bifunctional electrophiles has not been particularly successful. ONE-derived DNA adducts, which can arise only from lipid peroxidation, have now been characterized in the tissues of mouse models (8). Therefore, it might eventually be possible to detect these specific lipid hydroperoxide-derived DNA adducts after they have been excised from the DNA and excreted in the urine (see Fig. 2).
FIGURE 1.
Structures of lipid hydroperoxide-derived bifunctional electrophiles.
FIGURE 2.
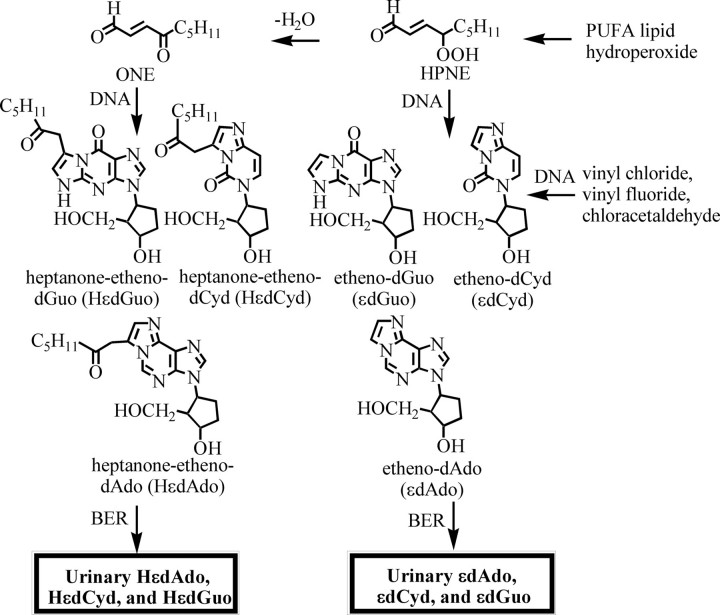
Formation of εDNA and HεDNA adducts through homolytic decomposition of lipid hydroperoxides. BER, base excision repair.
Oxidative Stress and Lipid Peroxidation
Oxidative stress represents one of the major cellular responses to toxic insults, carcinogenic chemicals, and environmental agents such as viruses and mycotoxins (9). During oxidative stress, there is a decrease in the amount of intracellular GSH and an increase in GSSG (10) with a concomitant increase in ROS and reactive nitrogen species (9) and an increase in the activity of COX-2 (11) and LOXs (10), involved in lipid peroxidation (1). ROS are generated constantly in vivo by a variety of endogenous processes, including normal mitochondrial aerobic respiration, phagocytosis of bacteria- or virus-containing cells, and peroxisome-mediated degradation of fatty acids (9). Increased ROS production occurs in inflammation; during radiation; or during metabolism of hormones, drugs, and environmental toxins. This can overwhelm endogenous protective mechanisms and increase ROS-mediated damage to GSH, proteins, DNA, and lipids. The resulting ROS-derived lipid hydroperoxides can then undergo homolytic decomposition to various bifunctional electrophiles that form DNA adducts with different lipid modifications (1–3;5). Alternatively, the bifunctional electrophiles can arise from homolytic decomposition of COX- and LOX-derived lipid hydroperoxides (11–13).
Intracellular lipid hydroperoxides are detoxified by GSH peroxidase-mediated reduction to lipid alcohols, with GSH providing the reducing equivalents (4). Peroxidized phospholipids are detoxified by peroxiredoxin 6 to the corresponding phospholipid alcohols in a GSH-dependent manner (14). GSH also readily forms adducts with lipid hydroperoxide-derived bifunctional electrophiles, providing an additional protective pathway (4, 12, 15). Formation of the GSH adducts is generally facilitated by GSTs, and the resulting adducts are exported from cells by ATP-binding and non-ATP-binding cassette transporters (10). Total GSH and GSSG concentrations are modulated in cells by low extracellular concentrations of lipid hydroperoxide-derived bifunctional electrophiles such as HNE. This can increase GSH concentrations by up-regulation of γ-glutamylcysteine ligase, a key enzyme involved in GSH biosynthesis. In contrast, high concentrations of bifunctional electrophiles deplete GSH (4) through a direct GST-mediated reaction as well as by inhibition of enzymes involved in GSH biosynthesis. GSH/GSSG homeostasis plays an important role in maintaining cellular redox status, and changes of the half-cell reduction potential of the 2GSH/GSSG couple correlate with the biological status of the cell (10).
It is difficult to accurately quantify intracellular GSH and GSSG concentrations because of the ease with which free sulfhydryl groups are oxidized during the lysis of cells. To overcome this problem, a stable isotope dilution LC-MRM-MS method was developed using 4-fluoro-7-sulfamoylbenzofurazan derivatization of GSH (10). Increased lipid peroxidation resulting from transfection of the human 15-LOX-1 gene into a mouse macrophage cell line was found to increase intracellular GSH biosynthesis. This resulted in a lower resting cellular redox potential and provided protection against exogenous lipid hydroperoxide-derived bifunctional electrophiles. Therefore, increased intracellular lipid peroxidation can induce a protective adaptive response (10).
Formation of Lipid Hydroperoxides
ROS-mediated oxidation of esterified LA-containing lipids and free LA results in the formation of four HPODE isomers as enantiomeric pairs. They are subsequently reduced to the corresponding hydroxyoctadecadienoic acids. Similarly, ROS-mediated oxidation of AA-containing lipids and free AA results in the formation of a complex mixture of HPETEs that are reduced to racemic hydroxyeicosatetraenoic acids (HETEs), including (15S)- and (15R)-HETE (11, 12, 16). Lipid hydroperoxides can also be formed by the action of LOXs (9) and COXs (17) on PUFAs.
High sensitivity, stable isotope dilution, normal phase, chiral LC-electron capture APCI-MS/MS methodology (18) makes it possible to separate and quantify all of the enantiomers and regioisomers of the PUFA alcohols derived from LA and AA (19, 20). Eicosanoids and isoprostanes can be quantified at the same time. This method revealed that endogenous (15S)-HPETE (as measured by (15S)-HETE release) was formed in amounts that were similar to prostaglandin E2 in unstimulated rat intestinal epithelial cells stably expressing COX-2 (RIES cells) (21). When exogenous AA was added to the RIES cells, the profile of eicosanoids was found to be dependent upon the time of incubation as well as the AA concentration (12). Interestingly, COX-2-mediated biosynthesis of prostaglandin E2 and (15S)-HETE was found to increase linearly with the addition of increasing amounts of AA to the cells. From the intercepts on the y axes of the regression lines, it was possible to calculate the amount of each eicosanoid formed from endogenous AA in resting cells. The calculated values were very close to the experimentally determined values. It was also possible to estimate how much endogenous AA (0.56 μm) had been mobilized by phospholipases in the unstimulated RIES cells. The ability to determine how much of an eicosanoid metabolite is formed in the absence of exogenous AA stimulation is particularly useful when endogenous production of the metabolite is below the detection limit of the assay that is being utilized.
Decomposition of Lipid Hydroperoxides to DNA-reactive Bifunctional Electrophiles
Studies on DNA adducts resulting from lipid hydroperoxide-derived bifunctional electrophiles have relied heavily on the use of reversed-phase LC-ESI-MS methodology (3). However, many of the bifunctional electrophiles themselves are poorly ionized under ESI conditions. Aldehydic bifunctional electrophiles can be converted to oxime derivatives to improve ESI efficiency. However, this results in syn- and anti-oxime isomers together with extremely complex LC chromatograms (22). Therefore, normal phase LC-APCI-MS was employed to quantify the lipid hydroperoxide-derived bifunctional electrophiles (21, 23, 24). HNE was more readily ionized than the other bifunctional electrophiles, so its APCI response was almost an order of magnitude more intense compared with the other bifunctional electrophiles. HPNE, trans-EDE, and ONE were the major products identified from the Fe(II)-mediated homolytic decomposition of (13S)-HPODE (24). The possible formation of ONE and HNE from HPNE was confirmed by treating HPNE with increasing concentrations of Fe(II). Vitamin C was more than twice as efficient at initiating the decomposition of 13-HPODE to bifunctional electrophiles compared with transition metal ions. It also induced the conversion of HPNE to ONE and HNE. Normal phase LC-APCI-MS methodology also showed that ONE was a major product from both Fe(II)-and vitamin C-mediated homolytic decomposition of (15S)-HPETE (23).
Formation of Lipid Hydroperoxide-derived DNA Adducts
A number of DNA adducts arising from lipid hydroperoxide-derived bifunctional electrophiles (Fig. 1) have been identified by LC-MS (3). The reaction between (13S)-HPODE and dGuo was shown to result in the formation of a single εDNA adduct, HεdGuo (Fig. 2) (25). The initially formed ethano adducts arose from highly regioselective nucleophilic addition of N-2 of the dGuo to the C-1 aldehyde of ONE, followed by reaction of N-1 at C-2 of the resulting α,β-unsaturated ketone. HεdAdo DNA adducts were also detected in the reaction between dAdo and 13-HPODE-derived ONE (Fig. 2) (26, 27). Furthermore, the reaction between ONE and dCyd resulted in formation of HεdCyd (Fig. 2) as a major product (28).
The endogenous formation of unsubstituted εDNA adducts was suggested originally to arise from lipid hydroperoxide-mediated epoxidation of HNE to 2,3-epoxy-4-hydroxynonanal and subsequent reaction of this bifunctional electrophile with DNA bases (29). Subsequently, it was suggested that this reaction was unlikely to occur in cells because of the relatively high pKa of the lipid hydroperoxides (30). Unsubstituted etheno adducts were also formed from the reaction of lipid hydroperoxide-derived DDE with dAdo or dGuo in the presence of peroxides (31). This pathway of endogenous εDNA adduct formation would require intracellular epoxidation of DDE to occur more rapidly than its detoxification by GSTs and aldoketoreductases (32). Unsubstituted etheno adducts were formed when DNA bases were treated with EDE and HPNE (Fig. 2) (33, 34). However, HPNE was much more reactive to DNA bases compared with EDE. Based on this increased reactivity, it appears that HPNE is most likely the major lipid hydroperoxide-derived bifunctional electrophile responsible for the formation of unsubstituted εDNA adducts (Fig. 2) (34). Previous studies have shown that environmental chemicals such as vinyl chloride, vinyl fluoride, and chloroethylene oxide form the same unsubstituted εDNA adducts (Fig. 2) (35, 36). Therefore, unsubstituted εDNA adducts do not arise solely from lipid peroxidation.
It has been proposed that a Hock rearrangement of 13-HPODE would lead to the intermediate formation of 12-oxo-(9Z)-dodecenoic acid, a carboxylate analog of (3Z)-nonenal (37). (3Z)-Nonenal is known to rapidly form HPNE, which in turn is converted to ONE and HNE (23, 24). This suggested that the ONE-related molecule, DODE (Fig. 1), might also be formed from the homolytic decomposition of 13-HPODE. Subsequently, DODE was synthesized and shown to be the 13-HPODE-derived bifunctional electrophile responsible for the formation of Cε-containing DNA adducts (38). Surprisingly, equimolar amounts of HεdGuo and CεdGuo were formed from the homolytic decomposition of (13S)-HPODE in the presence of dGuo. An analogous study showed that the carboxylate-containing bifunctional electrophile 5,8-dioxo-(10E)-octenoic acid was a major product arising from the homolytic decomposition of (5S)-HPETE and that it formed carboxylate-containing DNA adducts (13).
Analysis of Lipid Hydroperoxide-derived DNA Adducts in Biological Fluids
Substantial efforts have been made over the last 2 decades to develop MS methodology for the quantification of etheno- and propano-DNA adducts formed from covalent binding of lipid hydroperoxide-derived bifunctional electrophiles to DNA (1, 2, 5, 8, 11, 30, 39–52). MDA (Fig. 1) is one of the most intensively studied of the lipid hydroperoxide-derived bifunctional electrophiles that cause DNA damage (5, 39–42, 44, 50, 53). However, the cyclic propano-DNA adduct it forms with dGuo (M1dG) arises primarily in vivo from base propenals that are generated from ROS-mediated damage to the sugar backbone of DNA (52). M1dG can also be formed from MDA released during thromboxane A2 biosynthesis. Although M1dG is not a specific marker of lipid hydroperoxide-mediated DNA adduct formation, it may be a useful biomarker of endogenous DNA damage resulting from oxidative stress. M1dG has been detected in the liver DNA of humans (39) and animal models (40) as well as in circulating human leukocytes (44). Intriguingly, M1dG is excreted into rat urine as the corresponding 6-oxo metabolite (54). Unfortunately, the endogenous levels of 6-oxo-M1dG in human urine will probably to be too low to assess the amount of base propenal- and MDA-mediated DNA damage (50). However, this important finding raises the possibility that other lipid hydroperoxide-derived DNA adducts might undergo metabolism before they are excreted in the urine.
Stable isotope dilution GC-electron capture negative chemical ionization-MS methodology was used to determine the levels of ethenoadenine adducts in human placental DNA (46). A subsequent study reported the use of LC-MS/MS, which made it possible to analyze the intact DNA adduct without having to remove the 2′-deoxyribose sugar or having to prepare an electron-capturing derivative (47). Using this sensitive and specific LC-MS/MS methodology, it was shown that etheno-dAdo was in fact present at much lower levels in human placental DNA than found by the GC-MS assay. The discrepancy between the GC-electron capture negative chemical ionization-MS and LC-MS/MS studies was suggested to result from artifactual formation of etheno-dAdo during the isolation and derivatization procedure. This serves to highlight the difficulties associated with high sensitivity determinations of lipid hydroperoxide-derived DNA adducts in human tissue samples.
COX-2 can convert AA into (15S)-HPETE, which undergoes intracellular reduction to (15S)-HETE (4, 14). In settings of oxidative stress, where reducing pathways are compromised, we reasoned that (15S)-HPETE might survive long enough to undergo homolytic decomposition to DNA-reactive bifunctional electrophiles. Notably, COX-2 is localized to the nuclear membrane, which maximizes the potential translocation of any bifunctional electrophiles into the nucleus. Using RIES cells, which stably express COX-2, it was possible to show that basal endogenous production of (15S)-HPETE resulted in the formation of HεdGuo adducts (11). As predicted from in vitro studies (21, 23), there was a dose-dependent increase in endogenous HεdGuo adduct formation when vitamin C was added to the RIES cells (11). HεdGuo adduct formation and (15S)-HPETE biosynthesis were both inhibited by a specific COX-2 inhibitor and by aspirin.
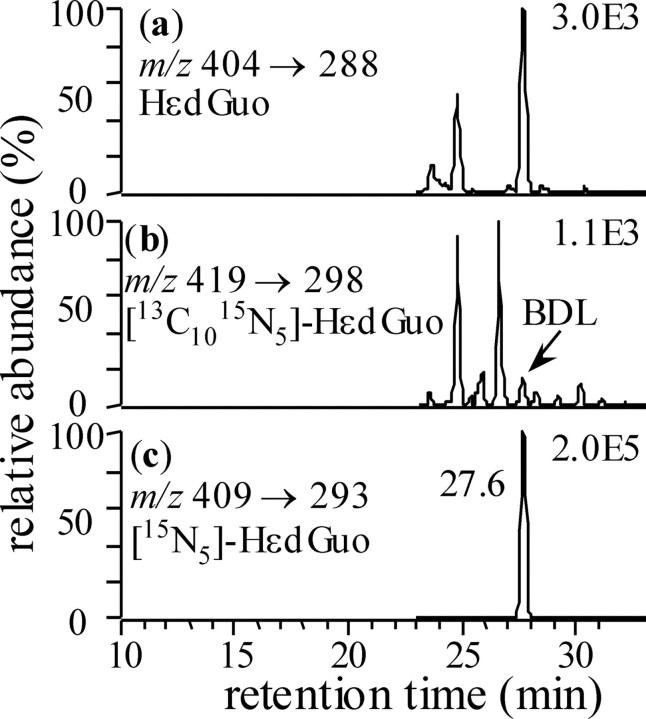
The role of COX-2 induced DNA damage in vivo was studied using the Min mouse model of colon carcinogenesis (8). The expressed dominant-negative 95-kDa truncated APC protein in Min mice is thought to prevent processing of β-catenin. This results in increased nuclear β-catenin, which, together with T-cell factor, activates expression of COX-2. Therefore, Min mice provide a useful model to explore the consequences of increased in vivo COX-2 expression. DNA was isolated from the entire small intestines of C57BL/6J and C57BL/6JAPCmin mice and then hydrolyzed in the presence of 15N5 -or 15N3-labeled internal standards. Stable isotope dilution LC-ESI-MRM-MS was conducted to provide maximal sensitivity and specificity. In separate experiments, it was shown that the HεDNA adducts were not generated as artifacts during isolation and hydrolysis by adding [13C,15N]DNA (Fig. 3). The use of labeled DNA provides a general method to confirm whether an endogenous DNA adduct can arise artifactually during isolation and hydrolysis of DNA or during LC-MS analysis.
FIGURE 3.
Use of totally stable isotope-labeled [13C,15N]DNA to detect artifactual formation of DNA adducts during sample analysis. The chromatograms show stable isotope dilution LC-MRM-MS analysis of DNA adducts from the small intestine of a Min mouse to which [13C,15N]DNA (520 μg) had been added before DNA extraction and hydrolysis. Ion chromatograms are shown for endogenous HεdGuo (a), [13C10,15N5]HεdGuo (from isotope-labeled DNA) (b), and [15N5]HεdGuo (internal standard) (c). BDL, below detection limit.
HεdGuo was increased from 0.6 adducts/107 normal bases in wild-type type mice to 1.8 adducts/107 normal bases in Min mice. HεdCyd was much less abundant. This suggested that base excision repair favored HεdAdo and HεdCyd over HεdGuo in the DNA and that the excised adducts might be excreted in the urine. Interestingly, no CεDNA adducts were detected, which suggests that peroxidation of LA did not contribute to DNA damage and/or that DODE was too polar to access the nuclear compartment. In keeping with this latter possibility, DODE could translocate across the plasma membrane only when esterified (32).
Conclusion
The existence of DNA repair pathways that have evolved to remove adducts arising from endogenous mutagens has been considered strong evidence in favor of their biological relevance to evolution (53). Because DNA is efficiently repaired, very little of the total DNA damage results in permanent mutations (7). This means that DNA adducts, which are repaired and appear in the urine, can be used as surrogate biomarkers of DNA damage. Urinary HεdAdo, HεdGuo, and HεdCyd, which are formed exclusively by lipid hydroperoxide-mediated DNA damage, might eventually be useful urinary biomarkers (Fig. 2). It is anticipated that immunoaffinity purification in combination with high sensitivity stable isotope dilution LC-MRM-MS will be required for the specific quantification of urinary HεDNA adducts (48, 50). Finally, the finding that HεdCyd is highly mutagenic in human cells (55) suggests that the quantification of urinary HεDNA adducts might allow the identification of human populations that are at risk for cancer through lipid peroxidation-mediated DNA damage.
This work was supported, in whole or in part, by National Institutes of Health Grants R01CA091016, U01ES016004, and P30ES013508. This is the seventh article of seven in the Oxidized Lipids Minireview Series. This minireview will be reprinted in the 2008 Minireview Compendium, which will be available in January, 2009.
Footnotes
The abbreviations used are: PUFA, polyunsaturated fatty acid; DDE, trans, trans-2,4-decadienal; EDE, 4,5-epoxy-(2E)-decenal; HNE, 4-hydroxy-(2E)-nonenal; HPNE, 4-hydroperoxy-(2E)-nonenal; MDA, malondialdehyde; ONE, 4-oxo-(2E)-nonenal; DODE, 9,12-dioxo-(10E)-dodecenoic acid; MS, mass spectrometry; ROS, reactive oxygen species; COX, cyclooxygenase; LOX, lipoxygenase; GST, glutathione S-transferase; LC-MRM-MS, liquid chromatography-multiple reaction monitoring-mass spectrometry; LA, linoleic acid; HPODE, hydroperoxy-(9Z),(11E)-octadecadienoic acid; AA, arachidonic acid; HPETE, hydroperoxyeicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid; APCI, atmospheric pressure chemical ionization; MS/MS, tandem MS; ESI, electrospray ionization; dGuo, 2′-deoxyguanosine; εDNA, etheno-DNA; Hε, heptanone-etheno; dAdo, 2′-deoxyadenosine; dCyd, 2′-deoxycytidine; Cε, carboxynonanone-etheno; GC, gas chromatography.
References
- 1.Blair, I. A. (2001) Exp. Gerontol. 36 1473–1481 [DOI] [PubMed] [Google Scholar]
- 2.Lee, S. H., and Blair, I. A. (2001) Trends Cardiovasc. Med. 11 148–155 [DOI] [PubMed] [Google Scholar]
- 3.Blair, I. A. (2005) in Encyclopedia of Mass Spectrometry (Caprioli, R. M., and Gross, M. L., eds) Vol. 3, pp. 283–307, Elsevier Ltd., Oxford [Google Scholar]
- 4.Blair, I. A. (2006) Curr. Drug Metab. 7 853–872 [DOI] [PubMed] [Google Scholar]
- 5.Marnett, L. J., Riggins, J. N., and West, J. D. (2003) J. Clin. Investig. 111 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kensler, T. W., Qian, G. S., Chen, J. G., and Groopman, J. D. (2003) Nat. Rev. Cancer 3 321–329 [DOI] [PubMed] [Google Scholar]
- 7.Sharma, R. A., and Farmer, P. B. (2004) Clin. Cancer Res. 10 4901–4912 [DOI] [PubMed] [Google Scholar]
- 8.Williams, M. V., Lee, S. H., Pollack, M., and Blair, I. A. (2006) J. Biol. Chem. 281 10127–10133 [DOI] [PubMed] [Google Scholar]
- 9.Ames, B. N., Shigenaga, M. K., and Hagen, T. M. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 7915–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu, P., Oe, T., and Blair, I. A. (2008) Rapid Commun. Mass Spectrom. 22 432–440 [DOI] [PubMed] [Google Scholar]
- 11.Lee, S. H., Williams, M. V., Dubois, R. N., and Blair, I. A. (2005) J. Biol. Chem. 280 28337–28346 [DOI] [PubMed] [Google Scholar]
- 12.Lee, S. H., Rangiah, K., Williams, M. V., Wehr, A. Y., Dubois, R. N., and Blair, I. A. (2007) Chem. Res. Toxicol. 20 1665–1675 [DOI] [PubMed] [Google Scholar]
- 13.Jian, W., Lee, S. H., Arora, J. S., Silva Elipe, M. V., and Blair, I. A. (2005) Chem. Res. Toxicol. 18 599–610 [DOI] [PubMed] [Google Scholar]
- 14.Manevich, Y., and Fisher, A. B. (2005) Free Radic. Biol. Med. 38 1422–1432 [DOI] [PubMed] [Google Scholar]
- 15.Jian, W., Lee, S. H., Mesaros, C., Oe, T., Silva Elipe, M. V., and Blair, I. A. (2007) Chem. Res. Toxicol. 20 1008–1018 [DOI] [PubMed] [Google Scholar]
- 16.Brash, A. R. (1999) J. Biol. Chem. 274 23679–23682 [DOI] [PubMed] [Google Scholar]
- 17.Porter, N. A., Caldwell, S. E., and Mills, K. A. (1995) Lipids 30 277–290 [DOI] [PubMed] [Google Scholar]
- 18.Singh, G., Gutierrez, A., Xu, K., and Blair, I. A. (2000) Anal. Chem. 72 3007–3013 [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. H., Williams, M. V., Dubois, R. N., and Blair, I. A. (2003) Rapid Commun. Mass Spectrom. 17 2168–2176 [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. H., and Blair, I. A. (2007) Methods Enzymol. 433 159–174 [DOI] [PubMed] [Google Scholar]
- 21.Lee, S. H., Oe, T., and Blair, I. A. (2001) Science 292 2083–2086 [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. H., and Blair, I. A. (2000) Chem. Res. Toxicol. 13 698–702 [DOI] [PubMed] [Google Scholar]
- 23.Williams, M. V., Lee, S. H., and Blair, I. A. (2005) Rapid Commun. Mass Spectrom. 19 849–858 [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. H., Oe, T., Arora, J. S., and Blair, I. A. (2005) J. Mass Spectrom. 40 661–668 [DOI] [PubMed] [Google Scholar]
- 25.Rindgen, D., Nakajima, M., Wehrli, S., Xu, K., and Blair, I. A. (1999) Chem. Res. Toxicol. 12 1195–1204 [DOI] [PubMed] [Google Scholar]
- 26.Rindgen, D., Lee, S. H., Nakajima, M., and Blair, I. A. (2000) Chem. Res. Toxicol. 13 846–852 [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. H., Rindgen, D., Bible, R. H., Jr., Hajdu, E., and Blair, I. A. (2000) Chem. Res. Toxicol. 13 565–574 [DOI] [PubMed] [Google Scholar]
- 28.Pollack, M., Oe, T., Lee, S. H., Silva Elipe, M. V., Arison, B. H., and Blair, I. A. (2003) Chem. Res. Toxicol. 16 893–900 [DOI] [PubMed] [Google Scholar]
- 29.Sodum, R. S., and Chung, F. L. (1991) Cancer Res. 51 137–143 [PubMed] [Google Scholar]
- 30.Douki, T., Odin, F., Caillat, S., Favier, A., and Cadet, J. (2004) Free Radic. Biol. Med. 37 62–70 [DOI] [PubMed] [Google Scholar]
- 31.Loureiro, A. P., Di, M. P., Gomes, O. F., and Medeiros, M. H. (2000) Chem. Res. Toxicol. 13 601–609 [DOI] [PubMed] [Google Scholar]
- 32.Jian, W., Arora, J. S., Oe, T., Shuvaev, V. V., and Blair, I. A. (2005) Free Radic. Biol. Med. 39 1162–1176 [DOI] [PubMed] [Google Scholar]
- 33.Lee, S. H., Oe, T., and Blair, I. A. (2002) Chem. Res. Toxicol. 15 300–304 [DOI] [PubMed] [Google Scholar]
- 34.Lee, S. H., Arora, J. A., Oe, T., and Blair, I. A. (2005) Chem. Res. Toxicol. 18 780–786 [DOI] [PubMed] [Google Scholar]
- 35.Barbin, A., Friesen, M., O'Neill, I. K., Croisy, A., and Bartsch, H. (1986) Chem. Biol. Interact. 59 43–54 [DOI] [PubMed] [Google Scholar]
- 36.Swenberg, J. A., Bogdanffy, M. S., Ham, A., Holt, S., Kim, A., Morinello, E. J., Ranasinghe, A., Scheller, N., and Upton, P. B. (1999) in Exocyclic DNA Adducts in Mutagenesis and Carcinogenesis (Singer, B., and Bartsch, H., eds) Volume 150, pp. 29–43, IARC Science Publications, Lyon, France [PubMed] [Google Scholar]
- 37.Uchida, K. (2003) Prog. Lipid Res. 42 318–343 [DOI] [PubMed] [Google Scholar]
- 38.Lee, S. H., Silva Elipe, M. V., Arora, J. S., and Blair, I. A. (2005) Chem. Res. Toxicol. 18 566–578 [DOI] [PubMed] [Google Scholar]
- 39.Chaudhary, A. K., Nokubo, M., Reddy, G. R., Yeola, S. N., Morrow, J. D., Blair, I. A., and Marnett, L. J. (1994) Science 265 1580–1582 [DOI] [PubMed] [Google Scholar]
- 40.Chaudhary, A. K., Nokubo, M., Marnett, L. J., and Blair, I. A. (1994) Biol. Mass Spectrom. 23 457–464 [DOI] [PubMed] [Google Scholar]
- 41.Chaudhary, A. J., Nokubo, M., Oglesby, T. D., Marnett, L. J., and Blair, I. A. (1995) J. Mass Spectrom. 30 1157–1166 [Google Scholar]
- 42.Chaudhary, A. K., Reddy, G. R., Blair, I. A., and Marnett, L. J. (1996) Carcinogenesis 17 1167–1170 [DOI] [PubMed] [Google Scholar]
- 43.Müller, M., Belas, F. J., Blair, I. A., and Guengerich, F. P. (1997) Chem. Res. Toxicol. 10 242–247 [DOI] [PubMed] [Google Scholar]
- 44.Rouzer, C. A., Chaudhary, A. K., Nokubo, M., Ferguson, D. M., Reddy, G. R., Blair, I. A., and Marnett, L. J. (1997) Chem. Res. Toxicol. 10 181–188 [DOI] [PubMed] [Google Scholar]
- 45.Ham, A. J., Ranasinghe, A., Morinello, E. J., Nakamura, J., Upton, P. B., Johnson, F., and Swenberg, J. A. (1999) Chem. Res. Toxicol. 12 1240–1246 [DOI] [PubMed] [Google Scholar]
- 46.Chen, H. J., Chiang, L. C., Tseng, M. C., Zhang, L. L., Ni, J., and Chung, F. L. (1999) Chem. Res. Toxicol. 12 1119–1126 [DOI] [PubMed] [Google Scholar]
- 47.Doerge, D. R., Churchwell, M. I., Fang, J. L., and Beland, F. A. (2000) Chem. Res. Toxicol. 13 1259–1264 [DOI] [PubMed] [Google Scholar]
- 48.Roberts, D. W., Churchwell, M. I., Beland, F. A., Fang, J. L., and Doerge, D. R. (2001) Anal. Chem. 73 303–309 [DOI] [PubMed] [Google Scholar]
- 49.Churchwell, M. I., Beland, F. A., and Doerge, D. R. (2002) Chem. Res. Toxicol. 15 1295–1301 [DOI] [PubMed] [Google Scholar]
- 50.Hoberg, A. M., Otteneder, M., Marnett, L. J., and Poulsen, H. E. (2004) J. Mass Spectrom. 39 38–42 [DOI] [PubMed] [Google Scholar]
- 51.Chen, H. J., and Chang, C. M. (2004) Chem. Res. Toxicol. 17 963–971 [DOI] [PubMed] [Google Scholar]
- 52.Zhou, X., Taghizadeh, K., and Dedon, P. C. (2005) J. Biol. Chem. 280 25377–25382 [DOI] [PubMed] [Google Scholar]
- 53.Marnett, L. J. (2000) Carcinogenesis 21 361–370 [DOI] [PubMed] [Google Scholar]
- 54.Knutson, C. G., Wang, H., Rizzo, C. J., and Marnett, L. J. (2007) J. Biol. Chem. 282 36257–36264 [DOI] [PubMed] [Google Scholar]
- 55.Pollack, M., Yang, I. Y., Kim, H. Y., Blair, I. A., and Moriya, M. (2006) Chem. Res. Toxicol. 19 1074–1079 [DOI] [PubMed] [Google Scholar]