Abstract
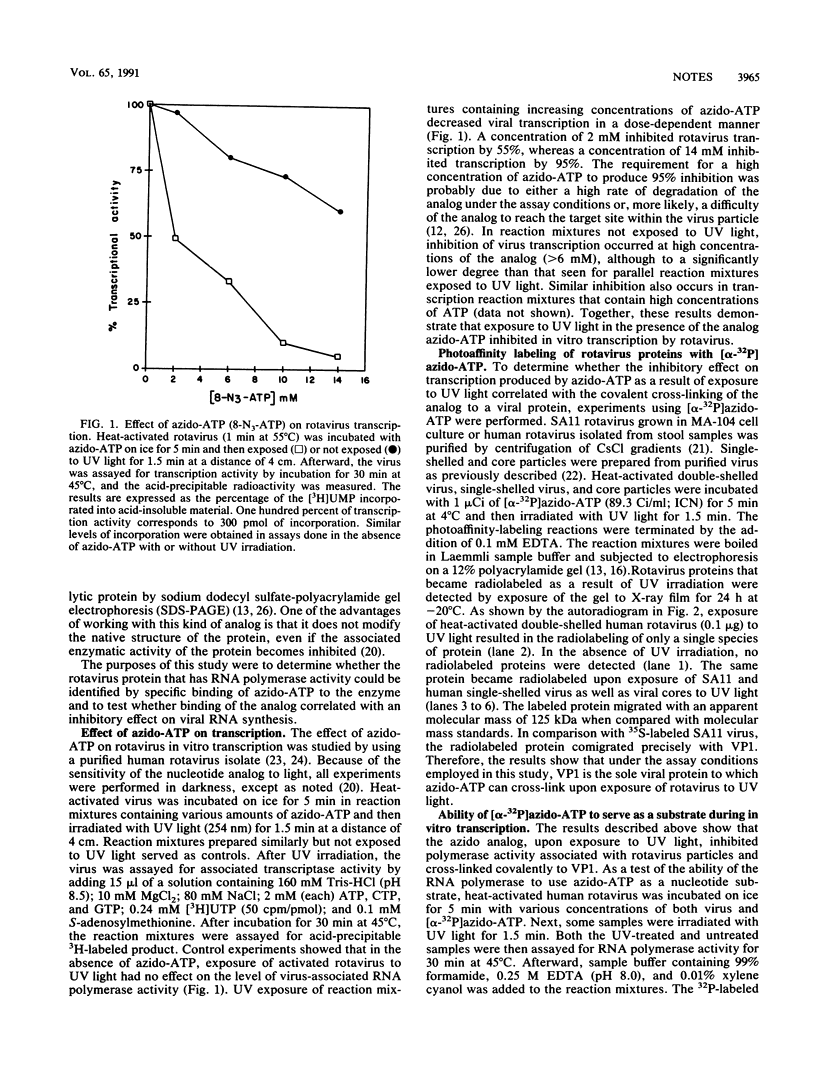
Rotavirus single-shelled particles have several enzymatic activities that are involved with the synthesis of capped mRNAs both in vivo and in vitro. Because single-shelled particles must be structurally intact to carry out transcription, it has proven to be difficult to identify the protein within such particles that possesses associated RNA polymerase activity. One approach for characterizing the function of the individual proteins within single-shelled particles is to use nucleotide analogs to specifically label those proteins, such as the viral RNA polymerase, that have affinity for nucleotides. In this study, 8-azido-ATP (azido-ATP), a photoreactable nucleotide analog, was used to identify the viral RNA polymerase on the basis of the ability of the analog to inhibit transcription activity associated with rotavirus particles on exposure to UV light. When single-shelled particles were treated with UV light in the presence of [alpha-32P]azido-ATP, the structural protein VP1 became radiolabeled because of cross-linking of the nucleotide analog, and there was a corresponding decrease in the ability of the particles to synthesize mRNA. In parallel experiments in which single-shelled particles were not exposed to UV light, VP1 was not radiolabeled and the particles successfully used azido-ATP as a substrate for the synthesis of viral mRNAs. Taken together, these results are consistent only with the conclusion that VP1 is the rotavirus RNA-dependent RNA polymerase.
Full text
PDF


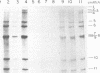
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C. F., López S., Espejo R. T. Gene protein products of SA11 simian rotavirus genome. J Virol. 1982 Jan;41(1):42–50. doi: 10.1128/jvi.41.1.42-50.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bican P., Cohen J., Charpilienne A., Scherrer R. Purification and characterization of bovine rotavirus cores. J Virol. 1982 Sep;43(3):1113–1117. doi: 10.1128/jvi.43.3.1113-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Charpilienne A., Chilmonczyk S., Estes M. K. Nucleotide sequence of bovine rotavirus gene 1 and expression of the gene product in baculovirus. Virology. 1989 Jul;171(1):131–140. doi: 10.1016/0042-6822(89)90519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Nishikawa K., Gorziglia M., Kapikian A. Z. Nucleotide sequence of gene segment 1 of a porcine rotavirus strain. Virology. 1989 Dec;173(2):743–749. doi: 10.1016/0042-6822(89)90590-4. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Yalamanchili R., Raengsakulrach B., Baumann R. P., Staczek J., O'Callaghan D. J. Viral transcripts in cells infected with defective interfering particles of equine herpesvirus type 1. Virology. 1989 Sep;172(1):1–10. doi: 10.1016/0042-6822(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Helmberger-Jones M., Patton J. T. Characterization of subviral particles in cells infected with simian rotavirus SA11. Virology. 1986 Dec;155(2):655–665. doi: 10.1016/0042-6822(86)90225-4. [DOI] [PubMed] [Google Scholar]
- Imai M., Akatani K., Ikegami N., Furuichi Y. Capped and conserved terminal structures in human rotavirus genome double-stranded RNA segments. J Virol. 1983 Jul;47(1):125–136. doi: 10.1128/jvi.47.1.125-136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K. Structure and function of the reovirus genome. Microbiol Rev. 1981 Dec;45(4):483–501. doi: 10.1128/mr.45.4.483-501.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julin D. A., Lehman I. R. Photoaffinity labeling of the recBCD enzyme of Escherichia coli with 8-azidoadenosine 5'-triphosphate. J Biol Chem. 1987 Jul 5;262(19):9044–9051. [PubMed] [Google Scholar]
- Kumar A., Charpilienne A., Cohen J. Nucleotide sequence of the gene encoding for the RNA binding protein (VP2) of RF bovine rotavirus. Nucleic Acids Res. 1989 Mar 11;17(5):2126–2126. doi: 10.1093/nar/17.5.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. B., Both G. W. Completion of the genomic sequence of the simian rotavirus SA11: nucleotide sequences of segments 1, 2, and 3. Virology. 1990 Jul;177(1):324–331. doi: 10.1016/0042-6822(90)90487-c. [DOI] [PubMed] [Google Scholar]
- Owens J. R., Haley B. E. A study of adenosine 3'-5' cyclic monophosphate binding sites of human erythrocyte membranes using 8-azidoadenosine 3'-5' cyclic monophosphate, a photoaffinity probe. J Supramol Struct. 1976;5(1):91–102. doi: 10.1002/jss.400050110. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Kochman M. Photoaffinity labeling of rabbit muscle fructose-1,6-bisphosphate aldolase with 8-azido-1,N6-ethenoadenosine 5'-triphosphate. Biochemistry. 1987 Jun 16;26(12):3466–3471. doi: 10.1021/bi00386a033. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Pizarro J. L., Sandino A. M., Pizarro J. M., Fernández J., Spencer E. Characterization of rotavirus guanylyltransferase activity associated with polypeptide VP3. J Gen Virol. 1991 Feb;72(Pt 2):325–332. doi: 10.1099/0022-1317-72-2-325. [DOI] [PubMed] [Google Scholar]
- Potter R. L., Haley B. E. Photoaffinity labeling of nucleotide binding sites with 8-azidopurine analogs: techniques and applications. Methods Enzymol. 1983;91:613–633. doi: 10.1016/s0076-6879(83)91054-6. [DOI] [PubMed] [Google Scholar]
- Sandino A. M., Jashes M., Faúndez G., Spencer E. Role of the inner protein capsid on in vitro human rotavirus transcription. J Virol. 1986 Nov;60(2):797–802. doi: 10.1128/jvi.60.2.797-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandino A. M., Pizarro J., Fernández J., Fellay M. C., Spencer E. Involvement of structural and nonstructural polypeptides on rotavirus RNA synthesis. Arch Biol Med Exp (Santiago) 1988 Dec;21(3-4):381–392. [PubMed] [Google Scholar]
- Spencer E., Arias M. L. In vitro transcription catalyzed by heat-treated human rotavirus. J Virol. 1981 Oct;40(1):1–10. doi: 10.1128/jvi.40.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E., García B. I. Effect of S-adenosylmethionine on human rotavirus RNA synthesis. J Virol. 1984 Oct;52(1):188–197. doi: 10.1128/jvi.52.1.188-197.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody A. Y., Vader C. R., Woody R. W., Haley B. E. Photoaffinity labeling of DNA-dependent RNA polymerase from Escherichia coli with 8-azidoadenosine 5'-triphosphate. Biochemistry. 1984 Jun 19;23(13):2843–2848. doi: 10.1021/bi00308a001. [DOI] [PubMed] [Google Scholar]