Abstract
Motor control demands coordinated excitation and inhibition across distributed brain neuronal networks. Recent work has suggested that multiple sclerosis (MS) may be associated with impairments of neuronal inhibition, as part of more general progressive impairments of connectivity. Here we report results from a prospective, multi-centre fMRI study designed to characterise the changes in patients relative to healthy controls during a simple cued hand movement task. This study was conducted at eight European sites using 1.5 Tesla scanners. Brain deactivation during right hand movement was assessed in 56 right-handed patients with relapsing-remitting or secondary progressive MS without clinically-evident hand impairment and in 60 age-matched, healthy subjects. The MS patients showed reduced task-associated deactivation relative to healthy controls in the pre- and postcentral gyri of the ipsilateral hemisphere in the region functionally specialised for hand movement control. We hypothesise that this impairment of deactivation is related to deficits of transcallosal connectivity and GABAergic neurotransmission occurring with the progression of pathology in the MS patients. This study has substantially extended previous observations with a well-powered, multicentre study. The clinical significance of these deactivation changes is still uncertain, but the functional anatomy of the affected region suggests that they could contribute to impairments of motor control.
Keywords: fMRI, multiple sclerosis, movement, inhibitory neurotransmission
Introduction
Extensive characterisation of functional activation in response to unilateral hand tapping tasks has provided evidence for potentially adaptive relative increases in regional brain activation (including the primary sensorimotor and premotor cortices ipsilateral to the hand moved) in MS patients with respect to healthy controls (Filippi et al., 2002; Filippi et al., 2004a; Filippi et al., 2004b; Lee et al., 2000; Pantano et al., 2002a; Reddy et al., 2000a; Reddy et al., 2000b; Rocca et al., 2005; Rocca et al., 2002a). These studies have identified clear functional correlations with diffuse neuronal damage throughout the motor system suggesting a relationship between the burden of pathology and the neurophysiological changes. Related studies of cognitive functions in MS patients have demonstrated that abnormal neuronal activation patterns also are found in other functional systems (Au Duong et al., 2005; Audoin et al., 2003; Cader et al., 2006).
More recently, attention has been directed towards deactivation phenomena. For example, healthy controls are known to deactivate the ipsilateral motor cortex during unilateral hand tapping, a process that is likely mediated by interhemispheric inhibition (Allison et al., 2000). In a pilot study performed by us in a small group of patients, we found that when MS patients perform the same task, the negative BOLD signal is relatively reduced; a net positive BOLD response (activation) often can be observed (Manson et al., 2006).
Evidence suggests that the negative BOLD signal reflects a relative suppression of neuronal firing (Shmuel et al., 2006). Thus, our previous results imply an impairment of inhibition in the motor network in the brain of MS patients. This hypothesis is consistent with considerable evidence for transcallosal fibre dysfunction or loss (Cader et al., 2007) and with microarray studies of post-mortem material showing a deficit in expression of proteins associated with GABAergic neurotransmission (Dutta et al., 2006).
Here we report a substantial extension of our earlier pilot study using data available from a prospective, multi-centre fMRI study. The primary aim of this study was to more powerfully test the earlier observation. The multi-centre design also allowed us to explore the potential of multi-centre studies for elucidation of such phenomenona.
Methods
Subjects and Centres
78 patients and 81 control subjects were scanned at eight European sites. The sites comprised of the Department of Radiology, VU University Medical Centre, Amsterdam (Netherlands); MR Unit, Hospital Vall d’Hebron, Barcelona (Spain); Neurology/Neuroradiology Department, University of Basel, Basel (Switzerland); MR Research Unit, Medical University Graz, Graz (Austria); NMR Unit, Institute of Neurology, University College London, London (UK); Neuroimaging Research Unit, University Ospedale San Raffaele Milan, Milan (Italy); Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, University of Oxford, Oxford (UK); and Department of Neurological and Behavioral Sciences, University of Siena, Siena (Italy).
The inclusion criteria for this study required all subjects to be right-handed, non-smokers, and aged between 19– 55 years. In addition, patients included in the study had to fulfil the following criteria: relapsing-remitting or secondary progressive MS, no relapse or corticosteroids within the previous 3 months prior to scanning, Expanded Disability Status Scale (EDSS) score of ≤7.5 (Kurtzke 1983) and no complaint or clinical history of right hand impairment.
22 patients were excluded from the final analysis. 16 patients were excluded as they did not meet the original inclusion criteria (older than 55 years [2 patients], relapse within the last 3 months [4 patients], incorrect clinical subtype [2 patients with primary progressive MS, 6 patients with clinically isolated syndromes], left-handed [2 patients]). One patient was excluded because the patent was not fully compliant with the protocol. Data from a further 5 patients were excluded because of severe artefacts in the functional MRI data (EPI “ghosting” and/or unusually large signal loss due to B0 inhomogeneity). 21 healthy control subjects also were excluded from the analysis. fMRI data of 6 control cases were not included due to moderately severe artefacts in the functional data. 15 further control cases were excluded in order to optimally match control subjects to MS patients for age, sex and centre of enrolment.
After these exclusions from the original study population, 60 healthy control subjects and 56 patients with clinically definite MS were included in the analysis. The 60 right-handed, age-matched healthy subjects included 32 men and 28 women (median age 30 years, range 19–48 years) (see supplementary material). All of the patients (21 men, 35 women; median age 35 year, range 20–53 years) had clinically definite MS (relapsing-remitting or secondary progressive; median disease duration 6.7 years, range 1–21 years) according to the Poser criteria (Poser et al., 1983) ) (see supplementary material). Disability was assessed with Kurtzke Expanded Disability Status Scale (EDSS) (median EDSS 2.0, range 0–7.5) (Kurtzke 1983) at the time of the fMRI scanning by experienced neurologists. None of the patients had clinically evident visual deficits or impairment of right-hand movement on routine neurological examination. None of the patients had experienced a relapse or received corticosteroids within 3 months prior to scanning. All subjects were non-smokers and were advised to abstain from drinking caffeine-containing beverages for 12 h before the scan.
MRI scanning
MRI scans at all centres were performed on magnets with a field strength of 1.5 Tesla (Amsterdam: Siemens Sonata, Barcelona: Siemens Symphony Maestro Class, Basel: Siemens Sonata, Graz: Philips Intera, London: GE Signa Excite 11.0, Milan: Vision Siemens, Oxford: Siemens Sonata, Siena: Philips Gyroscan ACS-NT15). Sagital T1 weighted localiser images were acquired to identify the anterior-posterior commissural plane. Functional MRI data was obtained using a multi-slice gradient echo planar imaging (EPI) sequence (21 slices parallel to the anterior-posterior commissural plane, 3.75mm × 3.75mm × 6mm resolution, TE = 60ms, TR = 3000ms, FOV = 240×240, matrix = 64×64). Each fMRI scan lasted 6 minutes and consisted of 120 volumes, and each subject repeated the scan 4 times. High resolution T1-weighted anatomical scans were also acquired for registration of functional data (resolution of T1-weighted scans: 1mm × 0.5mm × 0.5mm for Barcelona, 1mm × 1mm × 1mm for Basel and Oxford, 1mm × 1.5mm × 1mm for Amsterdam and London, 1mm × 1mm × 3mm for Siena).
Experimental Design
The fMRI experiment was a “block” design, with six 30s periods of hand tapping alternating with six 30s periods of rest. This 6 minute sequence was repeated four times in each session. Subjects were asked to perform a repetitive flexion-extension of the last four fingers of the right hand moving together in time with a 1 Hz visual stimulus. All centres used a standardised frame to restrict the amplitude of motion to 3 cm. Additionally, all centres were provided with a standardised metronome equipped with a red flashing LED to pace movement at a frequency of 1 Hz. The LED was switched off during rest periods. Subjects were trained on the task before entering the scanner and were monitored during the scan to ensure the task was being performed correctly.
Image Analysis
The analysis was performed using tools from the FMRIB Software Library (www.fmrib.ox.ac.uk/fsl). At the first level, which consists of each individual fMRI scan (4 per subject), the following were applied: motion correction (Jenkinson et al., 2002), spatial smoothing using a Gaussian kernel of full-width half-maximum 8mm, and nonlinear high-pass temporal filtering. Statistical analysis was parametric based on the general linear model. EPI images were registered to anatomical images and standard space using FLIRT with affine transformation and 12 degrees of freedom (Jenkinson and Smith, 2001). The second level analysis combined the four scans from each subject, in order to produce an average activation map for each individual.
A third level analysis was applied with mixed effects, where all individual patient data from the second level analysis was pooled and compared to the pooled data from healthy controls (Woolrich et al., 2004). The main effect of the task in healthy controls, age and centre were maintained as explanatory variables. At the third level, three different contrasts were examined in both patient and healthy control groups: main deactivation effect of task, age-related fMRI deactivation, and deactivation in relation to centre. The main deactivation effect was calculated through a one-sample t-test. The effect of age was then incorporated by including it as an additional covariate, and centre effect was calculated through variance analysis (1 factor, 7 levels).
In order to compare patients with healthy controls, the contrasts of patients relative to controls and controls relative to patients were calculated for the main effect of task as an unpaired two-sample t-test).To test specifically for deactivation differences, a binary mask was prepared as the sum of deactivation (positive contrst of rest vs. movement blocks) for the patients and the controls. The relative magnitude of the group differences in deactivation between patients and controls was assessed only within this mask. The variability in patient-control contrast across centres was calculated using an ANOVA model. To account for centre variability within the group difference, an f-test was performed for 8 contrasts in this model, each contrast representing, for each of the 8 sites, the difference of controls-minus-patients minus the average across all sites of the difference controls-minus-patients. To determine the contrast of age effect in patients compared to age effect in healthy controls, a two-sample unpaired t-test was used with two group averages as explanatory variables, and the ages of each group were added as co-variates.
All Z-stat maps were corrected for multiple comparisons using cluster detection, with clusters defined by Z>2.3 and corrected cluster significance threshold of p<0.05 (Forman et al., 1995)
ROI Analysis
Region of interest (ROI) analysis was performed to test for individual local deactivation responses in the hand region (Yousry et al., 1997) of the ipsilateral motor cortex in standard MNI space by the following parameters: 26≤x≤42, −22≥y≥−34, 56≤z≤66. The ROI was applied to the second level COPE (contrast of parameter estimates, referring to the weighting parameters in the general linear model fits, a measure of the degree of “activation” in the contrast) maps of each subject and mean activation was calculated as the mean COPE within the ROI.
Results
Right hand tapping elicited activation (Z>2.3, corrected p<0.05) in a distributed network of cortical and subcortical regions associated with motor control in the 60 healthy subjects and in the 56 patients with MS, as has been well described in other reports (see e.g., Lee et al., 2000) (data not shown). We tested for relative deactivation (i.e., decrease in BOLD signal during hand-tapping relative to rest) associated with right hand tapping task for the healthy controls. We found significant deactivation in the ipsilateral sensorimotor cortex as well as more widely, most prominently including the anterior cingulate cortex and precuneus (Fig. 1).
Figure 1.
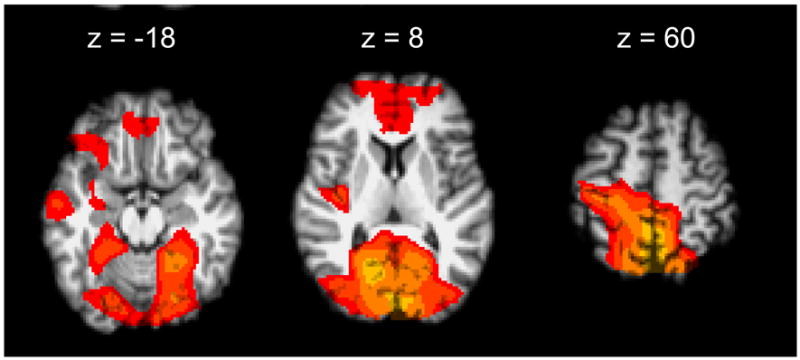
Healthy Control Deactivation. Deactivation was seen in the ipsilateral sensorimotor cortex and contralateral cerebellum as well as in areas outside of the motor network. (Z>2.3, cluster threshold p<0.05)
We then tested for statistically significant differences in deactivation between MS patients and the healthy controls in regions showing deactivation for the groups individually. We performed this with using two complementary mixed-effects contrasts: healthy controls relative to the MS patients, and MS patient relative to the healthy controls. Significantly greater deactivation was found for healthy controls in the ipsilateral (right) pre- and postcentral gyri with a primary cluster centre of gravity located at x=30, y= −29, z= 55, with additional, smaller clusters at x=6.5, y= −26, z=43, x=50, y=−53, z= 16 and x=−37, y= −82, z=5 (Fig 2). There were no regions of significantly greater deactivation for MS patients relative to the healthy controls.
Figure 2.
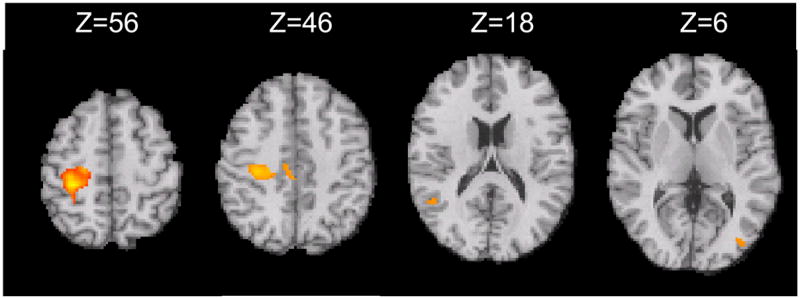
Healthy Control Deactivation relative to MS Patient Deactivation Contrast, masked for deactivation inhanges in MS include impaired deactivation, as well as the previously well-described c the groups. The deactivation cluster with the largest magnitude was located in the ipsilateral sensorimotor cortex (Z>2.3, cluster threshold p<0.05).
To estimate the extent of deactivation differences, we performed a region of interest (ROI) analysis in the ipsilateral motor cortex to determine the relative mean parameter estimates from the general linear model (COPE score, an index of the magnitude of signal change) (see Methods for ROI selection). We confirmed large decreases in the relative deactivation: controls −26 ± 29; MS patients, −12 ± 29 (p<0.01).
This was a multi-centre study, which raises the potential for introduction of centre-specific data biases. We therefore tested whether deactivation differences varied between individual centres. Only centre 1 showed significantly greater deactivation in the healthy controls this was limited to regions in the visual cortex and the brain stem (Fig. 3).
Figure 3.
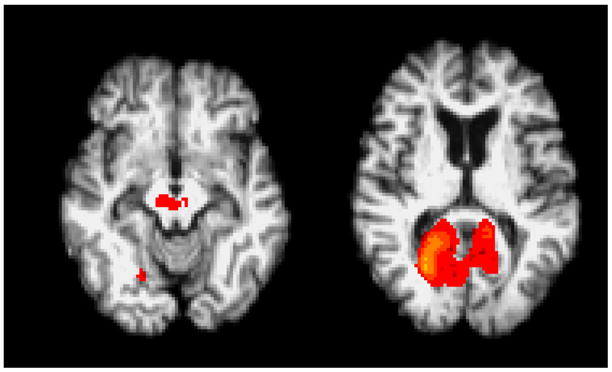
Centre Differences. The group mean activation image from centre 1 shows significant differences relative to other centres in the visual cortex and brain stem.
Using a separate statistical model, we found that age did not significantly explain any of the variation in relative deactivation with the task across the whole group. There was no correlation between relative deactivation in the ipsilateral primary sensorimotor cortex and disability (EDSS) for the patients (data not shown).
Discussion
Ipsilateral sensorimotor cortex deactivation with hand movement has previously been identified with relatively small numbers of subjects (Allison et al., 2000). Our first analysis confirmed with substantial study power that even a simple motor task is associated with clear fMRI deactivation in the healthy brain. FMRI deactivation in this context is most likely associated with relatively reduced local blood flow and oxygen extraction, consistent with a relative inhibitory response (Shmuel et al, 2006, Stefanovic et al., 2004). In some regions, such as the ipsilateral primary sensorimotor, this effect may be a consequence of transcallosal inhibition, as we have discussed previously (Manson et al., 2006). Our observations here illustrate more widespread deactivation than reported initially for a hand movement-related rest vs. movement contrast (Allison et al., 2000), as well. The anatomical distribution of changes is consistent with that previously reported by us (and others) to be associated with “resting state networks”, regions of coherent BOLD signal change suggesting functional integration, whose activity is increased in the unstimulated brain (rest) and decreased with stimulation or a task (DeLuca et al., 2006, Beckmann et al, 2005). Modulation of these activation areas is correlated with neuropsychological factors related to attentional focus (Mason et al, 2007; McKiernan et al., 2003).
With the increased power afforded by this multi-centre study, we have confirmed that MS patients have decreased movement-associated BOLD signal deactivation in the ipsilateral sensorimotor cortex, as we suggested previously in a small, pilot study (Manson et al., 2006). Lenzi and her colleagues also recently have shown a correlation between increased T1-lesion load (a marker of more axonally-destructive lesions) or increased mean diffusivity (consistent with greater axonal loss) and increased relative activation (a corollary of reduced deactivation) in the ipsilateral sensorimotor cortex with a simple hand movement task (Lenzi et al., 2007). Transcallosal fibres may be particularly vulnerable to injury with MS because of the preferential clustering of lesions in regions around the lateral ventricles (Lee et al., 1999). There is evidence for callosal fibre metabolic impairment, as well as loss (Cader et al., 2007). Impairment of deactivation also may be related to deficits in GABAergic neurotransmission (Dutta et al., 2006).
Inhibition (deactivation) is a critical control mechanism in neuronal networks. We postulate that the deactivation changes found here for the MS patients reflect functional consequences of pathology that also contribute to the abnormal activation patterns associated with the disease and to behavioural impairments (Filippi and Rocca, 2005; Filippi et al., 2002; Filippi et al., 2004a; Filippi et al., 2004b; Lee et al., 2000; Leocani et al., 2001; Pantano et al., 2002a; Pantano et al., 2002b; Pantano et al., 2005; Reddy et al., 2000a; Reddy et al., 2000b; Reddy et al., 2002b; Rocca et al., 2005; Rocca et al., 2002a; Rocca et al., 2004). Apparent increases in relative activation in the ipsilateral sensorimotor cortex therefore need not reflect adaptive responses (e.g., recruitment of ipsilateral corticospinal tract) as some of the previous work has suggested, but may simply be a reflection of functional pathological changes and potentially maladaptive.
A recent study has demonstrated a correlation between relative rostral anterior cingulate (ACC) deactivation and global grey matter atrophy with a delayed recognition task in patients with MS (Morgan et al., 2007). The absence of rostral ACC deactivation changes in our study could reflect differences in the patient populations. However, different neural circuits are engaged with the cognitive task. A different pattern of pathology-dependent relative deactivation may be expected even if similar mechanisms (e.g., reduced signal phase coherence with conduction defects, reduced information transfer with axonal loss) underlie both observations. A potentially attractive aspect of the transcallosal circuit is that the relatively well-defined functional anatomy simplifies analysis and interpretation of changes.
This study incidentally confirms that it is possible to perform meaningful multi-centre studies with MS patients in order to better characterise weaker signal changes. Because task-associated BOLD deactivation is typically of a smaller magnitude than BOLD activation, larger numbers of subjects are needed to adequately power a study relative to the numbers for an activation study. This was tested directly using the current data. When the relative deactivation contrast of healthy controls vs. MS patients was examined on a centre-by-centre basis, only the data from 3 out of 8 individual centres (mimicking the results that would be reported if these were considered as individual studies) would have showed statistically significant deactivation in the MS patients (SCM, PMM, personal observations). However, as described in the results, the data in aggregate suggest that this observation is a consequence of lack of statistical power to detect changes in any individual centre population, rather than meaningful centre-by-centre heterogeneity: in directly comparing the mean deactivation contrast (healthy controls vs. MS patients) of each individual centre with the combined mean deactivation contrast of the other centres, the only centre that significantly differed was Centre 1, where differences were found only in the visual cortex and the brain stem (in which the anatomy could not be well-defined). In a post-hoc exploration of differences between the stimuli at centres, we found that Centre 1 used a visual stimulus (large red circle projected on a screen) different from that at the other centres (small red LED).
A potential limitation of our study is that gender was not well-matched between patients and healthy controls. Sexual dimorphism of structural asymmetries in motor cortex suggests the potential for gender differences in functional activation (Amunts et al, 2000), as has been well-reported for some cognitive tasks (e.g., Bell et al. 2006). However, we are not aware of reports of differences in activation between men and women for a simple hand-tapping task and did not observe such differences in healthy control data (PMM, CW, unpublished observations).
In summary, here we have confirmed and extended our previous observation that brain functional changes in activation. The clinical significance of alternations in these broader response changes is uncertain at this point, but they could contribute directly to impairments of specificity of motor control (Manson et al., 2006).
Supplementary Material
Acknowledgments
PMM thanks the MRC (UK) and the MS Society of Great Britain and Northern Ireland for support. PMM is an employee of GlaxoSmithKline. The design and preparation of this review were done under the auspices of the European Community network for Magnetic Resonance research in MS (MAGNIMS).
Abbreviations
- EDSS
Extended Disability Status Score
- EPI
echoplanar image
- fMRI
functional MRI
- MNI
Montreal Neurological Institute
- PMd
dorsal premotor cortex
- ROI
region of interest
References
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–42. doi: 10.1212/wnl.54.1.135. [DOI] [PubMed] [Google Scholar]
- Amunts K, Janke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38:304–12. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Au Duong MV, Audoin B, Boulanouar K, Ibarrola D, Malikova I, Confort-Gouny S, et al. Altered functional connectivity related to white matter changes inside the working memory network at the very early stage of MS. J Cereb Blood Flow Metab. 2005;25:1245–53. doi: 10.1038/sj.jcbfm.9600122. [DOI] [PubMed] [Google Scholar]
- Audoin B, Ibarrola D, Ranjeva JP, Confort-Gouny S, Malikova I, Ali-Cherif A, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51–8. doi: 10.1002/hbm.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilan AH, Dave S, Silverstone PH. MAles and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30:529–38. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Cader S, Johansen-Berg H, Wylezinska M, Palace J, Behrens TE, Smith S, Matthews PM. Discordant white matter N-acetylasparate and diffusion MRI measures suggest that chronic metabolic dysfunction contributes to axonal pathology in multiple sclerosis. Neuroimage. 2007 May 15;36(1):19–27. doi: 10.1016/j.neuroimage.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain. 2006;129:527–37. doi: 10.1093/brain/awh670. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;15;29:1359–67. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–89. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA. MRI evidence for multiple sclerosis as a diffuse disease of the central nervous system. J Neurol. 2005;252 (Suppl 5):v16–24. doi: 10.1007/s00415-005-5004-5. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Falini A, Caputo D, Ghezzi A, Colombo B, et al. Correlations between structural CNS damage and functional MRI changes in primary progressive MS. Neuroimage. 2002;15:537–46. doi: 10.1006/nimg.2001.1023. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Mezzapesa DM, Falini A, Colombo B, Scotti G, et al. A functional MRI study of cortical activations associated with object manipulation in patients with MS. Neuroimage. 2004a;21:1147–54. doi: 10.1016/j.neuroimage.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Mezzapesa DM, Ghezzi A, Falini A, Martinelli V, et al. Simple and complex movement-associated functional MRI changes in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Hum Brain Mapp. 2004b;21:108–17. doi: 10.1002/hbm.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Moore P, Gee J, et al. Neural basis for verb processing in Alzheimer's disease: an fMRI study. Neuropsychology. 2003;17:658–74. doi: 10.1037/0894-4105.17.4.658. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lee M, Reddy H, Johansen-Berg H, Pendlebury S, Jenkinson M, Smith S, et al. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol. 2000;47:606–13. [PubMed] [Google Scholar]
- Lee MA, Smith S, Palace J, Narayanan S, Silver N, Minicucci L, Filippi M, Miller DH, Arnold DL, Matthews PM. Spatial mapping of T2 and gadolinium-enhancing T1 lesion volumes in multiple sclerosis: evidence for distinct mechanisms of lesion genesis? Brain. 1999 Jul;122(Pt 7):1261–70. doi: 10.1093/brain/122.7.1261. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Conte A, Mainero C, Fasca V, Fubelli F, Totaro P, Caramia F, Inghilleri M, Pozzilli C, Pantano P. Effects of corpus callusum damage on ipsilateral mtoor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp. 2007;28:636–44. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Colombo B, Magnani G, Martinelli-Boneschi F, Cursi M, Rossi P, et al. Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement--EEG evidence. Neuroimage. 2001;13:1186–92. doi: 10.1006/nimg.2001.0759. [DOI] [PubMed] [Google Scholar]
- MacKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:294–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Manson SC, Palace J, Frank JA, Matthews PM. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res. 2006;174:728–33. doi: 10.1007/s00221-006-0517-4. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007 Jan 19;315(5810):393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Sammer G, Courtney SM, Wolters T, Melchior H, Blecker CR, Oschmann P, Kaps M, Vaitl D. Distinct mechanisms of altered brain activation in patients with multiple sclerosis. Neuroimage. 2007;37:937–46. doi: 10.1016/j.neuroimage.2007.05.045. [DOI] [PubMed] [Google Scholar]
- Pantano P, Iannetti GD, Caramia F, Mainero C, Di Legge S, Bozzao L, et al. Cortical motor reorganization after a single clinical attack of multiple sclerosis. Brain. 2002a;125:1607–15. doi: 10.1093/brain/awf164. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Iannetti GD, Caramia F, Di Legge S, Piattella MC, et al. Contribution of corticospinal tract damage to cortical motor reorganization after a single clinical attack of multiple sclerosis. Neuroimage. 2002b;17:1837–43. doi: 10.1006/nimg.2002.1313. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Lenzi D, Caramia F, Iannetti GD, Piattella MC, et al. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128:2146–53. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, et al. Alzheimer's patients engage an alternative network during a memory task. Ann Neurol. 2005;58:870–9. doi: 10.1002/ana.20653. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The neural correlates of consciousness: an analysis of cognitive skill learning. Philos Trans R Soc Lond B Biol Sci. 1998;353:1889–901. doi: 10.1098/rstb.1998.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy H, Bendahan D, Lee MA, Johansen-Berg H, Donaghy M, Hilton-Jones D, et al. An expanded cortical representation for hand movement after peripheral motor denervation. J Neurol Neurosurg Psychiatry. 2002a;72:203–10. doi: 10.1136/jnnp.72.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Arnoutelis R, Jenkinson M, Antel J, Matthews PM, et al. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000a;123 ( Pt 11):2314–20. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Matthews PM, Hoge RD, Pike GB, Duquette P, et al. Relating axonal injury to functional recovery in MS. Neurology. 2000b;54:236–9. doi: 10.1212/wnl.54.1.236. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Woolrich M, Mitsumori T, Lapierre Y, Arnold DL, et al. Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain. 2002b;125:2646–57. doi: 10.1093/brain/awf283. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G, et al. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–26. doi: 10.1016/S1474-4422(05)70171-X. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Falini A, Colombo B, Scotti G, Comi G, Filippi M. Adaptive functional changes in the cerebral cortex of patients with nondisabling multiple sclerosis correlate with the extent of brain structural damage. Ann Neurol. 2002a;51:330–9. doi: 10.1002/ana.10120. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Gallo A, Colombo B, Falini A, Scotti G, Comi G, et al. Pyramidal tract lesions and movement-associated cortical recruitment in patients with MS. Neuroimage. 2004;23:141–7. doi: 10.1016/j.neuroimage.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Gavazzi C, Mezzapesa DM, Falini A, Colombo B, Mascalchi M, et al. A functional magnetic resonance imaging study of patients with secondary progressive multiple sclerosis. Neuroimage. 2003a;19:1770–7. doi: 10.1016/s1053-8119(03)00242-8. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Matthews PM, Caputo D, Ghezzi A, Falini A, Scotti G, et al. Evidence for widespread movement-associated functional MRI changes in patients with PPMS. Neurology. 2002b;58:866–72. doi: 10.1212/wnl.58.6.866. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Mezzapesa DM, Falini A, Ghezzi A, Martinelli V, Scotti G, et al. Evidence for axonal pathology and adaptive cortical reorganization in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage. 2003b;18:847–55. doi: 10.1016/s1053-8119(03)00043-0. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–9. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006 doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22:771–8. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120 ( Pt 1):141–57. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


