Abstract
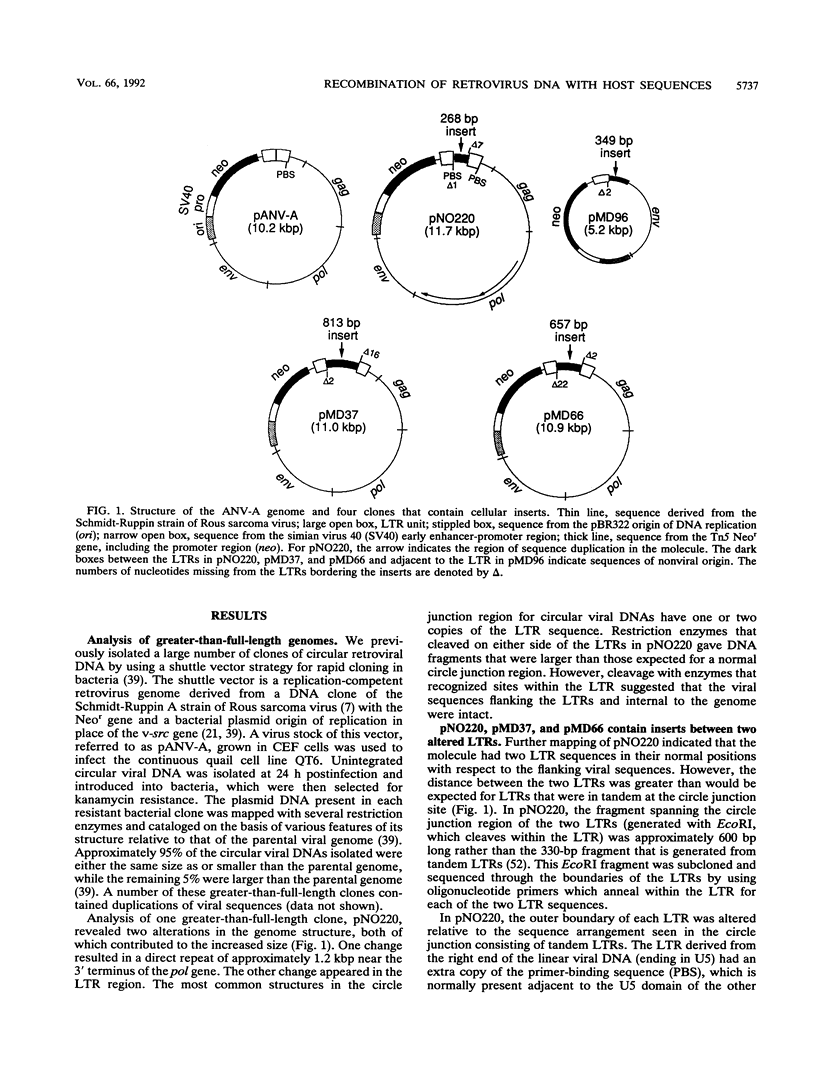
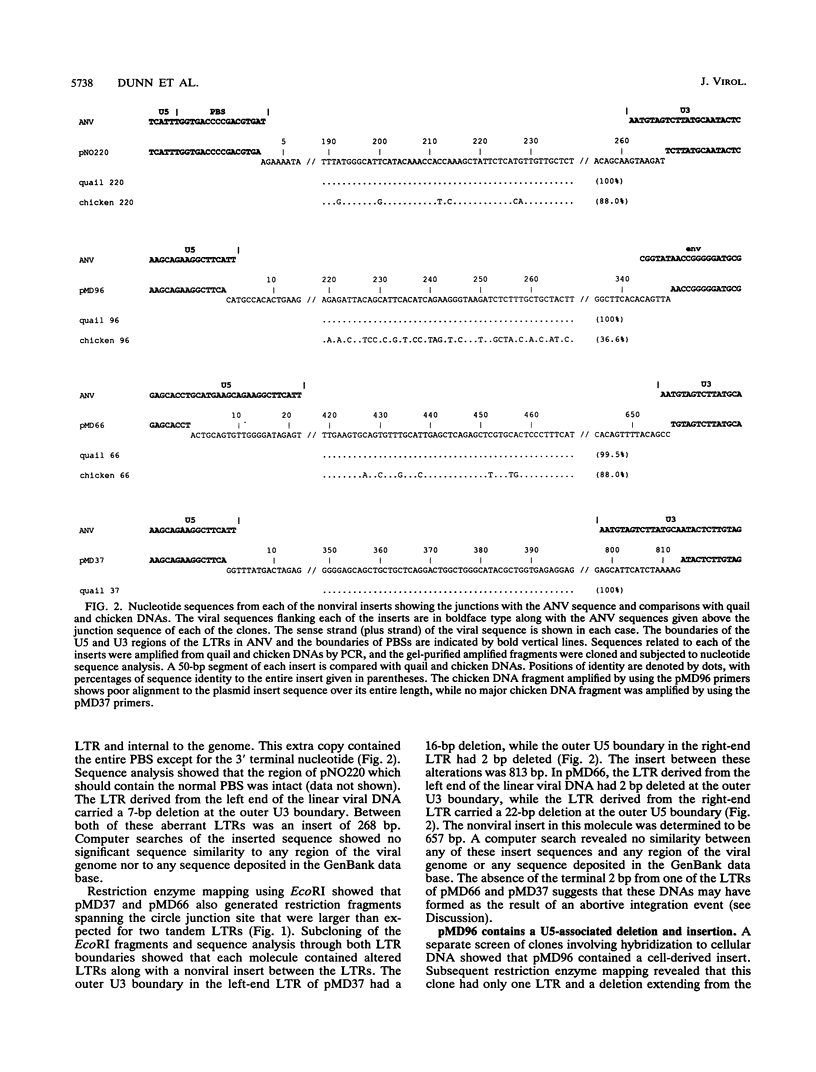
We have used a replication-competent shuttle vector based on the genome of Rous sarcoma virus to characterize genomic rearrangements that occur during retrovirus replication. The strategy involved cloning circular DNA that was generated during an acute infection. While analyzing a class of retroviral DNA clones that are greater than full length, we found several clones which had acquired nonviral inserts in positions adjacent to the long terminal repeats (LTRs). There appear to be two distinct mechanisms leading to the incorporation of cellular sequences into these clones. Three of the molecules contain a cell-derived insert at the circle junction site between two LTR units. Two of these molecules appear to be the results of abortive integration attempts, because of which, in each case, one of the LTRs is missing 2 bases at its junction with the cell-derived insert. In the third clone, pNO220, the cellular sequences are flanked by an inappropriately placed copy of the tRNA primer-binding site on one side and a partial copy of the U3 sequence as part of the LTR on the other side. A fourth molecule we characterized, pMD96, has a single LTR with a U5-bounded deletion of viral sequences spanning gag and pol, with cell-derived sequences inserted at the site of the deletion; its origin may be related mechanistically to pNO220. Sequence analysis indicates that all of the cellular inserts were derived from the cell line used for the acute infection rather than from sequences carried into the cell as part of the virus particle. Northern (RNA) analysis of cellular RNA demonstrated that the cell-derived sequences of two clones, pNO220 and pMD96, were expressed as polyadenylated RNA in uninfected cells. One mechanism for the joining of viral and cellular sequences suggested by the structures of pNO220 and pMD96 is recombination occurring during viral DNA synthesis, with cellular RNA serving as the template for the acquisition of cellular sequences.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Hunter T. Identification of a packaged cellular mRNA in virions of rous sarcoma virus. J Virol. 1981 Aug;39(2):471–480. doi: 10.1128/jvi.39.2.471-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Brown P. O. Integration of retroviral DNA. Curr Top Microbiol Immunol. 1990;157:19–48. doi: 10.1007/978-3-642-75218-6_2. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Cywinski A., Taylor J. M. Reverse transcription of 7S L RNA by an avian retrovirus. J Virol. 1985 May;54(2):278–284. doi: 10.1128/jvi.54.2.278-284.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derr L. K., Strathern J. N., Garfinkel D. J. RNA-mediated recombination in S. cerevisiae. Cell. 1991 Oct 18;67(2):355–364. doi: 10.1016/0092-8674(91)90187-4. [DOI] [PubMed] [Google Scholar]
- Gallis B., Linial M., Eisenman R. An avian oncovirus mutant deficient in genomic RNA: characterization of the packaged RNA as cellular messenger RNA. Virology. 1979 Apr 15;94(1):146–161. doi: 10.1016/0042-6822(79)90445-8. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Tabin C. J., Wang J. Y., Weinberg R., Baltimore D. Transfection of fibroblasts by cloned Abelson murine leukemia virus DNA and recovery of transmissible virus by recombination with helper virus. J Virol. 1982 Jan;41(1):271–285. doi: 10.1128/jvi.41.1.271-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Evidence that retroviral transduction is mediated by DNA not by RNA. Proc Natl Acad Sci U S A. 1990 May;87(9):3604–3608. doi: 10.1073/pnas.87.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral transduction of oncogenic sequences involves viral DNA instead of RNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3733–3737. doi: 10.1073/pnas.85.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubin G., Hill M. Monomer and multimer covalently closed circular forms of Rous sarcoma virus DNA. J Virol. 1979 Feb;29(2):799–804. doi: 10.1128/jvi.29.2.799-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S. A., Coffin J. M. Efficient packaging of readthrough RNA in ALV: implications for oncogene transduction. Science. 1987 May 15;236(4803):845–848. doi: 10.1126/science.3033828. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hay N., Bishop J. M. The role of RNA molecules in transduction of the proto-oncogene c-fps. Cell. 1986 Mar 28;44(6):935–940. doi: 10.1016/0092-8674(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Hagino-Yamagishi K., Kawai S., Yamamoto T., Toyoshima K. Activation of the cellular src gene by transducing retrovirus. Mol Cell Biol. 1986 Jul;6(7):2420–2428. doi: 10.1128/mcb.6.7.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Ross J., Leder P. An association between globin messenger RNA and 60S RNA derived from Friend leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1154–1158. doi: 10.1073/pnas.71.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Koyama T. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biological properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):147–153. doi: 10.1128/jvi.51.1.147-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Koyama T., Harada F., Kawai S. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biochemical properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):154–162. doi: 10.1128/jvi.51.1.154-162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Fung Y. K., Majors J. E., Bishop J. M., Varmus H. E. Synthesis of plus strands of retroviral DNA in cells infected with avian sarcoma virus and mouse mammary tumor virus. J Virol. 1981 Jan;37(1):127–138. doi: 10.1128/jvi.37.1.127-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Shank P. R., Bishop J. M., Varmus H. E. Identification and characterization of dimeric and trimeric circular forms of avian sarcoma virus-specific DNA. Virology. 1980 Jun;103(2):425–433. doi: 10.1016/0042-6822(80)90201-9. [DOI] [PubMed] [Google Scholar]
- Levine K. L., Steiner B., Johnson K., Aronoff R., Quinton T. J., Linial M. L. Unusual features of integrated cDNAs generated by infection with genome-free retroviruses. Mol Cell Biol. 1990 May;10(5):1891–1900. doi: 10.1128/mcb.10.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M. Creation of a processed pseudogene by retroviral infection. Cell. 1987 Apr 10;49(1):93–102. doi: 10.1016/0092-8674(87)90759-8. [DOI] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Martin P., Henry C., Ferre F., Bechade C., Begue A., Calothy C., Debuire B., Stehelin D., Saule S. Characterization of a myc-containing retrovirus generated by propagation of an MH2 viral subgenomic RNA. J Virol. 1986 Mar;57(3):1191–1194. doi: 10.1128/jvi.57.3.1191-1194.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Koyama T., Kawai S. Frequent segregation of more-defective variants from a Rous sarcoma virus packaging mutant, TK15. J Virol. 1987 Oct;61(10):3208–3213. doi: 10.1128/jvi.61.10.3208-3213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Koyama T., Kawai S. Unusual features of the leader sequence of Rous sarcoma virus packaging mutant TK15. J Virol. 1985 Sep;55(3):881–885. doi: 10.1128/jvi.55.3.881-885.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. C., Bova-Hill C., Grandgenett D. P., Quinn T. P., Manfredi J. P., Swanstrom R. Rearrangements in unintegrated retroviral DNA are complex and are the result of multiple genetic determinants. J Virol. 1990 Nov;64(11):5475–5484. doi: 10.1128/jvi.64.11.5475-5484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. C., Swanstrom R. A new pathway in the generation of defective retrovirus DNA. J Virol. 1985 Dec;56(3):779–789. doi: 10.1128/jvi.56.3.779-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak V. K., Temin H. M. Broad spectrum of in vivo forward mutations, hypermutations, and mutational hotspots in a retroviral shuttle vector after a single replication cycle: deletions and deletions with insertions. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6024–6028. doi: 10.1073/pnas.87.16.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli G. A., Temin H. M. Characterization of large deletions occurring during a single round of retrovirus vector replication: novel deletion mechanism involving errors in strand transfer. J Virol. 1991 Sep;65(9):4786–4797. doi: 10.1128/jvi.65.9.4786-4797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Maihle N. J., Moscovici C., Crittenden L., Kung H. J. Mechanism of c-erbB transduction: newly released transducing viruses retain poly(A) tracts of erbB transcripts and encode C-terminally intact erbB proteins. J Virol. 1988 Jul;62(7):2437–2443. doi: 10.1128/jvi.62.7.2437-2443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson D., Dugan D., Reddy E. P. Aberrant splicing events that are induced by proviral integration: implications for myb oncogene activation. Proc Natl Acad Sci U S A. 1987 May;84(10):3171–3175. doi: 10.1073/pnas.84.10.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Dieckmann M., Berg P. Transduction of cellular neo mRNA by retrovirus-mediated recombination. J Virol. 1990 Dec;64(12):5783–5796. doi: 10.1128/jvi.64.12.5783-5796.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A., Coffin J. M. Mechanism of transduction by retroviruses. Science. 1992 Feb 14;255(5046):841–845. doi: 10.1126/science.1371365. [DOI] [PubMed] [Google Scholar]
- Swain A., Coffin J. M. Polyadenylation at correct sites in genome RNA is not required for retrovirus replication or genome encapsidation. J Virol. 1989 Aug;63(8):3301–3306. doi: 10.1128/jvi.63.8.3301-3306.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Bishop J. M., Varmus H. E. Structure of a replication intermediate in the synthesis of Rous sarcoma virus DNA in vivo. J Virol. 1982 Apr;42(1):337–341. doi: 10.1128/jvi.42.1.337-341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cywinski A. A defective retrovirus particle (SE21Q1b) packages and reverse transcribes cellular RNA, utilizing tRNA-like primers. J Virol. 1984 Aug;51(2):267–271. doi: 10.1128/jvi.51.2.267-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]



