Abstract
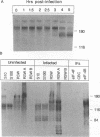
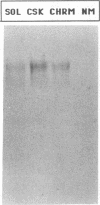
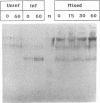
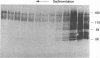
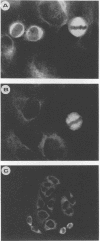
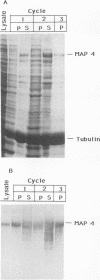
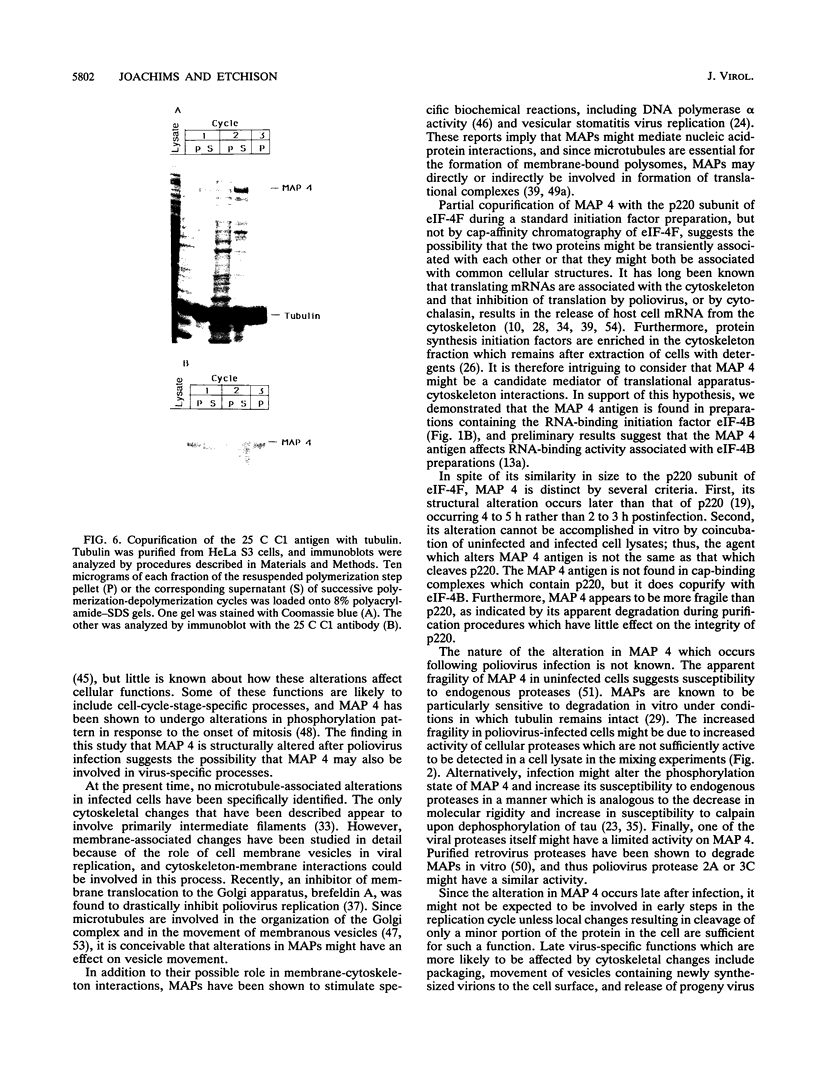
Poliovirus infection results in profound changes in cellular metabolism and architecture. To identify alterations in cellular proteins following poliovirus infection which might account for these changes, monoclonal antibodies were prepared by screening for differences in antigen pattern in infected and uninfected cell lysates. Further characterization of the antigen of one such antibody (25 C C1) is described in this report. The 25 C C1 antigen is a cytoskeleton-associated protein which decreases in size 4 to 5 h postinfection. It copurifies with some of the protein synthesis initiation factors but not with eucaryotic initiation factor (eIF)-4F, the p220 subunit of which is cleaved following infection (D. Etchison, S. C. Milburn, I. Edery, N. Sonenberg, and J. W. B. Hershey, J. Biol. Chem. 257:14806-14810, 1982). Unlike alteration of p220, alteration of the 25 C C1 antigen is not due to a protease which can be detected by cell lysate mixing experiments. Alteration of the antigen occurs during purification, suggesting progressive proteolysis, but the alteration is more extensive in preparations from infected cells than in those from uninfected cells. A recombinant phage expressing the antigenic determinant was isolated from a human fibroblast cDNA library, and the sequence of the cDNA insert was found to be entirely contained within the established sequence of microtubule-associated protein (MAP) 4 (R. R. West, K. M. Tenbarge, and J. B. Olmsted, J. Biol. Chem. 266:21886-21896, 1991). The antigen distribution, as detected by indirect immunofluorescence, was similar to, but more diffuse than, the distribution of tubulin. The antibody recognized the largest abundant HeLa cell MAP, which copurified with tubulin after three cycles of polymerization-depolymerization, thus confirming the identity of the antigen as MAP 4. These results indicate that poliovirus infection of HeLa cells affects the structural integrity of a cytoskeletal protein, MAP 4.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Brown-Luedi M. L., Hershey J. W. Protein synthesis initiation factors from rabbit reticulocytes: purification, characterization, and radiochemical labeling. Methods Enzymol. 1979;60:15–35. doi: 10.1016/s0076-6879(79)60005-8. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983 Nov;131(1):39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Troxler M., Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990 Mar;64(3):1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Proteolysis of the p220 component of the cap-binding protein complex is not sufficient for complete inhibition of host cell protein synthesis after poliovirus infection. J Virol. 1987 Apr;61(4):986–991. doi: 10.1128/jvi.61.4.986-991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Microtubule-associated proteins from cultured HeLa cells. Analysis of molecular properties and effects on microtubule polymerization. J Biol Chem. 1980 Dec 10;255(23):11570–11576. [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Widespread distribution of a 210,000 mol wt microtubule-associated protein in cells and tissues of primates. J Cell Biol. 1980 Dec;87(3 Pt 1):802–808. doi: 10.1083/jcb.87.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C. Purification and assay of primate 210K microtubule-associated protein. Methods Enzymol. 1986;134:147–156. doi: 10.1016/0076-6879(86)34083-7. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969 Nov 14;166(3907):885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Clark M. E., Dasgupta A. A transcriptionally active form of TFIIIC is modified in poliovirus-infected HeLa cells. Mol Cell Biol. 1990 Oct;10(10):5106–5113. doi: 10.1128/mcb.10.10.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. E., Hämmerle T., Wimmer E., Dasgupta A. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 1991 Oct;10(10):2941–2947. doi: 10.1002/j.1460-2075.1991.tb07844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. Microtubule MAPping. Cell. 1990 Mar 9;60(5):701–702. doi: 10.1016/0092-8674(90)90083-q. [DOI] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- Davies M. V., Pelletier J., Meerovitch K., Sonenberg N., Kaufman R. J. The effect of poliovirus proteinase 2Apro expression on cellular metabolism. Inhibition of DNA replication, RNA polymerase II transcription, and translation. J Biol Chem. 1991 Aug 5;266(22):14714–14720. [PubMed] [Google Scholar]
- Etchison D., Ehrenfeld E. Viral polypeptides associated with the RNA replication complex in poliovirus-infected cells. Virology. 1980 Nov;107(1):135–142. doi: 10.1016/0042-6822(80)90279-2. [DOI] [PubMed] [Google Scholar]
- Etchison D., Etchison J. R. Monoclonal antibody-aided characterization of cellular p220 in uninfected and poliovirus-infected HeLa cells: subcellular distribution and identification of conformers. J Virol. 1987 Sep;61(9):2702–2710. doi: 10.1128/jvi.61.9.2702-2710.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Etchison D., Milburn S. Separation of protein synthesis initiation factor eIF4A from a p220-associated cap binding complex activity. Mol Cell Biochem. 1987 Jul;76(1):15–25. doi: 10.1007/BF00219394. [DOI] [PubMed] [Google Scholar]
- Etchison D., Smith K. Variations in cap-binding complexes from uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1990 May 5;265(13):7492–7500. [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- Hagestedt T., Lichtenberg B., Wille H., Mandelkow E. M., Mandelkow E. Tau protein becomes long and stiff upon phosphorylation: correlation between paracrystalline structure and degree of phosphorylation. J Cell Biol. 1989 Oct;109(4 Pt 1):1643–1651. doi: 10.1083/jcb.109.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill V. M., Summers D. F. A minor microtubule-associated protein is responsible for the stimulation of vesicular stomatitis virus transcription in vitro. J Gen Virol. 1990 Feb;71(Pt 2):289–298. doi: 10.1099/0022-1317-71-2-289. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Johnson G. V., Jope R. S., Binder L. I. Proteolysis of tau by calpain. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1505–1511. doi: 10.1016/0006-291x(89)91150-9. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Lara J., Wambach M. Nontranslated cellular mRNAs are associated with the cytoskeletal framework in influenza virus or adenovirus infected cells. Virology. 1989 Apr;169(2):312–322. doi: 10.1016/0042-6822(89)90156-6. [DOI] [PubMed] [Google Scholar]
- Keates R. A. Stabilization of microtubule protein in glycerol solutions. Can J Biochem. 1981 May;59(5):353–360. doi: 10.1139/o81-049. [DOI] [PubMed] [Google Scholar]
- Kliewer S., Muchardt C., Gaynor R., Dasgupta A. Loss of a phosphorylated form of transcription factor CREB/ATF in poliovirus-infected cells. J Virol. 1990 Sep;64(9):4507–4515. doi: 10.1128/jvi.64.9.4507-4515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Litersky J. M., Johnson G. V. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J Biol Chem. 1992 Jan 25;267(3):1563–1568. [PubMed] [Google Scholar]
- Matus A., Green G. D. Age-related increase in a cathepsin D like protease that degrades brain microtubule-associated proteins. Biochemistry. 1987 Dec 15;26(25):8083–8086. doi: 10.1021/bi00399a010. [DOI] [PubMed] [Google Scholar]
- Maynell L. A., Kirkegaard K., Klymkowsky M. W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992 Apr;66(4):1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Tamm I. Incorporation of lipid precursors into cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):39–47. doi: 10.1016/0042-6822(72)90236-x. [DOI] [PubMed] [Google Scholar]
- Ornelles D. A., Fey E. G., Penman S. Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol Cell Biol. 1986 May;6(5):1650–1662. doi: 10.1128/mcb.6.5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parysek L. M., Asnes C. F., Olmsted J. B. MAP 4: occurrence in mouse tissues. J Cell Biol. 1984 Oct;99(4 Pt 1):1309–1315. doi: 10.1083/jcb.99.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parysek L. M., Wolosewick J. J., Olmsted J. B. MAP 4: a microtubule-associated protein specific for a subset of tissue microtubules. J Cell Biol. 1984 Dec;99(6):2287–2296. doi: 10.1083/jcb.99.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- Schimmel H., Traub P. The effect of mengovirus infection on lipid synthesis in cultured Ehrlich ascites tumor cells. Lipids. 1987 Feb;22(2):95–103. doi: 10.1007/BF02534860. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Neighbors B., McIntosh J. R., Salmon E. D. Isolation of microtubules and a dynein-like MgATPase from unfertilized sea urchin eggs. J Biol Chem. 1984 May 25;259(10):6516–6525. [PubMed] [Google Scholar]
- Schulman H., Kuret J., Jefferson A. B., Nose P. S., Spitzer K. H. Ca2+/calmodulin-dependent microtubule-associated protein 2 kinase: broad substrate specificity and multifunctional potential in diverse tissues. Biochemistry. 1985 Sep 24;24(20):5320–5327. doi: 10.1021/bi00341a008. [DOI] [PubMed] [Google Scholar]
- Shioda M., Okuhara K., Murofushi H., Mori A., Sakai H., Murakami-Murofushi K., Suzuki M., Yoshida S. Stimulation of DNA polymerase alpha activity by microtubule-associated proteins. Biochemistry. 1991 Dec 3;30(48):11403–11412. doi: 10.1021/bi00112a006. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Moskalewski S. Microtubules and the organization of the Golgi complex. Exp Cell Res. 1985 Jul;159(1):1–16. doi: 10.1016/s0014-4827(85)80032-x. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Centonze V. E., Peloquin J., Tombes R. M., Borisy G. G. Proteins of the mammalian mitotic spindle: phosphorylation/dephosphorylation of MAP-4 during mitosis. J Cell Sci. 1991 Apr;98(Pt 4):577–588. doi: 10.1242/jcs.98.4.577. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Whitfield J. F. Cytoplasmic microtubules are essential for the formation of membrane-bound polyribosomes. J Biol Chem. 1985 Jan 25;260(2):765–770. [PubMed] [Google Scholar]
- Wallin M., Deinum J., Goobar L., Danielson U. H. Proteolytic cleavage of microtubule-associated proteins by retroviral proteinases. J Gen Virol. 1990 Sep;71(Pt 9):1985–1991. doi: 10.1099/0022-1317-71-9-1985. [DOI] [PubMed] [Google Scholar]
- West R. R., Tenbarge K. M., Olmsted J. B. A model for microtubule-associated protein 4 structure. Domains defined by comparisons of human, mouse, and bovine sequences. J Biol Chem. 1991 Nov 15;266(32):21886–21896. [PubMed] [Google Scholar]
- Wood S. A., Park J. E., Brown W. J. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991 Nov 1;67(3):591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Fey E. G., Penman S., Stein J., Stein G. Multiple types of mRNA-cytoskeleton interactions. J Cell Biochem. 1990 Nov;44(3):177–187. doi: 10.1002/jcb.240440306. [DOI] [PubMed] [Google Scholar]