Abstract
The small envelope S protein of hepatitis B virus carrying the surface antigen has the unique property of mobilizing cellular lipids into empty envelope particles which are secreted from mammalian cells. We studied the biogenesis of such particles using site-directed mutagenesis. In this study, we describe the effect of deletions in the N-terminal hydrophobic and hydrophilic domains of the S protein. Whereas short overlapping deletions of hydrophilic sequences flanking the first hydrophobic domain were tolerated, larger deletions of the same sequences were not. Conversely, the hydrophilic region preceding the second hydrophobic domain was not permissive for even short deletions. Deletion of part or all of the first hydrophobic domain also completely blocked secretion, confirming that the entire apolar region serves an essential function. Most of the secretion-defective deletion mutants still entered the secretory pathway and translocated at least the second hydrophilic domain across the membrane of the endoplasmic reticulum. These mutants appeared to remain arrested in a membrane-associated configuration in the endoplasmic reticulum or the cis-Golgi compartment but preserved their capacity for oligomerization with the wild-type S protein. While secretion of wild-type S protein was specifically blocked by the formation of intracellularly retained mixed envelope aggregates, secretion of an unrelated protein (interleukin 9) was completely unaffected.
Full text
PDF


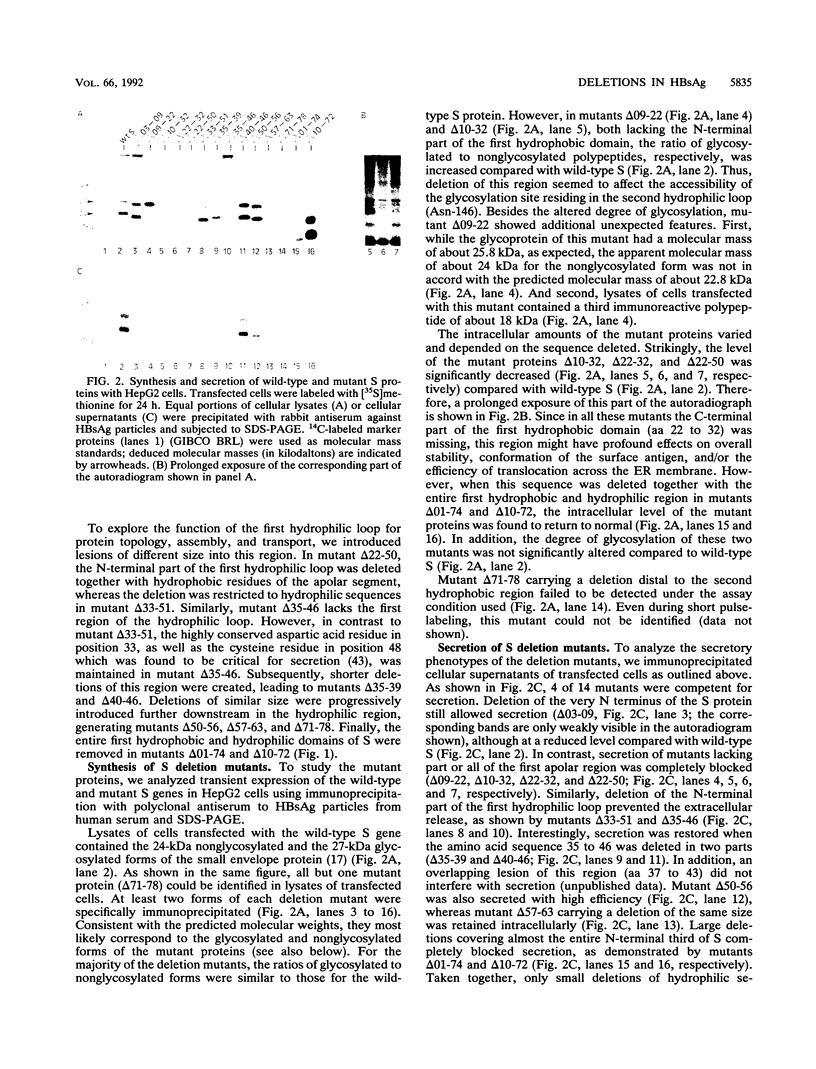




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki K., Shiosaki K., Araki M., Chisaka O., Matsubara K. The essential region for assembly and particle formation in hepatitis B virus surface antigen produced in yeast cells. Gene. 1990 May 14;89(2):195–201. doi: 10.1016/0378-1119(90)90006-d. [DOI] [PubMed] [Google Scholar]
- Armstrong J., Niemann H., Smeekens S., Rottier P., Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984 Apr 19;308(5961):751–752. doi: 10.1038/308751a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann B. J., Knowles W. J., Marchesi V. T. Synthetic peptides mimic the assembly of transmembrane glycoproteins. J Biol Chem. 1989 Mar 5;264(7):4033–4037. [PubMed] [Google Scholar]
- Boyd D., Beckwith J. The role of charged amino acids in the localization of secreted and membrane proteins. Cell. 1990 Sep 21;62(6):1031–1033. doi: 10.1016/0092-8674(90)90378-r. [DOI] [PubMed] [Google Scholar]
- Bruss V., Ganem D. Mutational analysis of hepatitis B surface antigen particle assembly and secretion. J Virol. 1991 Jul;65(7):3813–3820. doi: 10.1128/jvi.65.7.3813-3820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Thrift R. N., Wu C. C., Howell K. E. Apolipoprotein B is both integrated into and translocated across the endoplasmic reticulum membrane. Evidence for two functionally distinct pools. J Biol Chem. 1990 Jun 15;265(17):10005–10011. [PubMed] [Google Scholar]
- Delpeyroux F., Chenciner N., Lim A., Lambert M., Malpièce Y., Streeck R. E. Insertions in the hepatitis B surface antigen. Effect on assembly and secretion of 22-nm particles from mammalian cells. J Mol Biol. 1987 May 20;195(2):343–350. doi: 10.1016/0022-2836(87)90655-3. [DOI] [PubMed] [Google Scholar]
- Delpeyroux F., Chenciner N., Lim A., Malpièce Y., Blondel B., Crainic R., van der Werf S., Streeck R. E. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science. 1986 Jul 25;233(4762):472–475. doi: 10.1126/science.2425433. [DOI] [PubMed] [Google Scholar]
- Delpeyroux F., Van Wezel E., Blondel B., Crainic R. Structural factors modulate the activity of antigenic poliovirus sequences expressed on hybrid hepatitis B surface antigen particles. J Virol. 1990 Dec;64(12):6090–6100. doi: 10.1128/jvi.64.12.6090-6100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol Cell Biol. 1986 May;6(5):1454–1463. doi: 10.1128/mcb.6.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. The N-terminal (pre-S2) domain of a hepatitis B virus surface glycoprotein is translocated across membranes by downstream signal sequences. J Virol. 1990 Mar;64(3):1414–1419. doi: 10.1128/jvi.64.3.1414-1419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., MacRae D. R., Lingappa V. R., Ganem D. Multiple topogenic sequences determine the transmembrane orientation of the hepatitis B surface antigen. Mol Cell Biol. 1987 Oct;7(10):3591–3601. doi: 10.1128/mcb.7.10.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Ganem D. Assembly of hepadnaviral virions and subviral particles. Curr Top Microbiol Immunol. 1991;168:61–83. doi: 10.1007/978-3-642-76015-0_4. [DOI] [PubMed] [Google Scholar]
- Gavilanes F., Gonzalez-Ros J. M., Peterson D. L. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. J Biol Chem. 1982 Jul 10;257(13):7770–7777. [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986 Nov;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kuroki K., Russnak R., Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989 Oct;9(10):4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E., Luckow V., Kennedy R. C., Dreesman G. R., Notvall L., Summers M. D. Expression and characterization of hepatitis B virus surface antigen polypeptides in insect cells with a baculovirus expression system. J Virol. 1989 Apr;63(4):1549–1557. doi: 10.1128/jvi.63.4.1549-1557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal and membrane anchor functions overlap in the type II membrane protein I gamma CAT. J Cell Biol. 1988 Jun;106(6):1813–1820. doi: 10.1083/jcb.106.6.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Flint N., Haeuptle M. T., Dobberstein B. Structural requirements for membrane assembly of proteins spanning the membrane several times. J Cell Biol. 1989 Nov;109(5):2013–2022. doi: 10.1083/jcb.109.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfalla G., Armbruster L., Dequin S., Bertolotti R. Construction of an EBNA-producing line of well-differentiated human hepatoma cells and of appropriate Epstein-Barr virus-based shuttle vectors. Gene. 1989 Mar 15;76(1):27–39. doi: 10.1016/0378-1119(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Marquardt O., Heermann K. H., Seifer M., Gerlich W. H. Cell-type dependent expression and secretion of hepatitis B virus pre-S1 surface antigen. Postgrad Med J. 1987;63 (Suppl 2):41–50. [PubMed] [Google Scholar]
- Mayer T., Tamura T., Falk M., Niemann H. Membrane integration and intracellular transport of the coronavirus glycoprotein E1, a class III membrane glycoprotein. J Biol Chem. 1988 Oct 15;263(29):14956–14963. doi: 10.1016/S0021-9258(18)68131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern K., Ehrmann M., Beckwith J. Decoding signals for membrane protein assembly using alkaline phosphatase fusions. EMBO J. 1991 Oct;10(10):2773–2782. doi: 10.1002/j.1460-2075.1991.tb07826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Rutter W. J. Regulation of secretion of the hepatitis B virus major surface antigen by the preS-1 protein. J Virol. 1987 Mar;61(3):782–786. doi: 10.1128/jvi.61.3.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks G. D., Lamb R. A. Topology of eukaryotic type II membrane proteins: importance of N-terminal positively charged residues flanking the hydrophobic domain. Cell. 1991 Feb 22;64(4):777–787. doi: 10.1016/0092-8674(91)90507-u. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A. Conversion of a class II integral membrane protein into a soluble and efficiently secreted protein: multiple intracellular and extracellular oligomeric and conformational forms. J Cell Biol. 1990 Apr;110(4):999–1011. doi: 10.1083/jcb.110.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Nakamura G. R., Simonsen C. C., Levinson A. D., Brands R. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J Virol. 1986 Jun;58(3):884–892. doi: 10.1128/jvi.58.3.884-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Nakamura G. R., Yaffe A. Intracellular transport and secretion of hepatitis B surface antigen in mammalian cells. J Virol. 1984 Aug;51(2):346–353. doi: 10.1128/jvi.51.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R. F. Protein localization and virus assembly at intracellular membranes. Curr Top Microbiol Immunol. 1991;170:67–106. doi: 10.1007/978-3-642-76389-2_3. [DOI] [PubMed] [Google Scholar]
- Prange R., Clemen A., Streeck R. E. Myristylation is involved in intracellular retention of hepatitis B virus envelope proteins. J Virol. 1991 Jul;65(7):3919–3923. doi: 10.1128/jvi.65.7.3919-3923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh O., Umeda M., Imai H., Tunoo H., Inoue K. Lipid composition of hepatitis B virus surface antigen particles and the particle-producing human hepatoma cell lines. J Lipid Res. 1990 Jul;31(7):1293–1300. [PubMed] [Google Scholar]
- Schmitt E., Beuscher H. U., Huels C., Monteyne P., van Brandwijk R., van Snick J., Ruede E. IL-1 serves as a secondary signal for IL-9 expression. J Immunol. 1991 Dec 1;147(11):3848–3854. [PubMed] [Google Scholar]
- Simon K., Lingappa V. R., Ganem D. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J Cell Biol. 1988 Dec;107(6 Pt 1):2163–2168. doi: 10.1083/jcb.107.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly J., Howard C. R., Zuckerman A. J. Hepatitis B polypeptide vaccine preparation in micelle form. Nature. 1981 Mar 5;290(5801):51–54. doi: 10.1038/290051a0. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Van Snick J., Goethals A., Renauld J. C., Van Roost E., Uyttenhove C., Rubira M. R., Moritz R. L., Simpson R. J. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40). J Exp Med. 1989 Jan 1;169(1):363–368. doi: 10.1084/jem.169.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Zerial M., Huylebroeck D., Garoff H. Foreign transmembrane peptides replacing the internal signal sequence of transferrin receptor allow its translocation and membrane binding. Cell. 1987 Jan 16;48(1):147–155. doi: 10.1016/0092-8674(87)90365-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]