Abstract
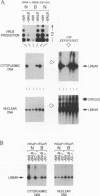
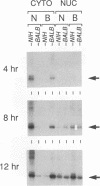
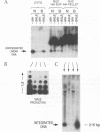
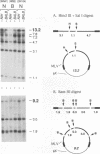
We have investigated the mechanisms by which alleles at the mouse Fv-1 locus restrict replication of murine leukemia viruses. Inhibition of productive infection is closely paralleled by reduced accumulation of integrated proviral DNA as well as by reduced levels of linear viral DNA in a cytoplasmic fraction. Nevertheless, viral DNA is present at nearly normal levels in a nuclear fraction, and total amounts of viral DNA are only mildly affected in restrictive infections, suggesting a block in integration to account for reduced levels of proviral DNA. However, integrase (IN)-dependent trimming of 3' ends of viral DNA occurs normally in vivo during restrictive infections, demonstrating that not all IN-mediated events are prevented in vivo. Furthermore, viral integration complexes present in nuclear extracts of infected restrictive cells are fully competent to integrate their DNA into a heterologous target in vitro. Thus, the Fv-1-dependent activity that restricts integration in vivo may be lost in vitro; alternatively, Fv-1 restriction may prevent a step required for integration in vivo that is bypassed in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone L. R., Myer F. E., Yang D. M., Ou C. Y., Koh C. K., Roberson L. E., Tennant R. W., Yang W. K. Reversal of Fv-1 host range by in vitro restriction endonuclease fragment exchange between molecular clones of N-tropic and B-tropic murine leukemia virus genomes. J Virol. 1983 Oct;48(1):110–119. doi: 10.1128/jvi.48.1.110-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Retroviral integration: structure of the initial covalent product and its precursor, and a role for the viral IN protein. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2525–2529. doi: 10.1073/pnas.86.8.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman F. D., Fujiwara T., Craigie R. Retroviral DNA integration directed by HIV integration protein in vitro. Science. 1990 Sep 28;249(4976):1555–1558. doi: 10.1126/science.2171144. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R. Fv-1 host restriction of Friend leukemia virus: analysis of unintegrated proviral DNA. J Virol. 1981 Oct;40(1):45–55. doi: 10.1128/jvi.40.1.45-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R., Kopchick J. Fv-1 host cell restriction of friend leukemia virus: microinjection of unintegrated viral DNA. J Virol. 1984 Apr;50(1):271–274. doi: 10.1128/jvi.50.1.271-274.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsky J., Soeiro R. Studies with aphidicolin on the Fv-1 host restriction of Friend murine leukemia virus. J Virol. 1982 Jul;43(1):182–190. doi: 10.1128/jvi.43.1.182-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R., Fujiwara T., Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990 Aug 24;62(4):829–837. doi: 10.1016/0092-8674(90)90126-y. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983 Dec;48(3):685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A. Analysis of mutant Moloney murine leukemia viruses containing linker insertion mutations in the 3' region of pol. J Virol. 1988 Nov;62(11):3958–3964. doi: 10.1128/jvi.62.11.3958-3964.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Varmus H. E. A mutant murine leukemia virus with a single missense codon in pol is defective in a function affecting integration. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6461–6465. doi: 10.1073/pnas.81.20.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttagupta S., Soeiro R. Host restriction of Friend leukemia virus: gag proteins of host range variants. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2320–2324. doi: 10.1073/pnas.78.4.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J., Bernstein A. Retrovirus vectors containing an internal attachment site: evidence that circles are not intermediates to murine retrovirus integration. J Virol. 1989 Jun;63(6):2844–2846. doi: 10.1128/jvi.63.6.2844-2846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman M., Bikel I., Figge J., Roberts T., Schlossman R., Livingston D. M. Localization of the simian virus 40 small t antigen in the nucleus and cytoplasm of monkey and mouse cells. J Virol. 1984 May;50(2):623–628. doi: 10.1128/jvi.50.2.623-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet C. M., Haseltine W. A. Circularization of human immunodeficiency virus type 1 DNA in vitro. J Virol. 1991 Dec;65(12):6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Temin H. M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977 Nov;24(2):461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Craigie R. Integration of mini-retroviral DNA: a cell-free reaction for biochemical analysis of retroviral integration. Proc Natl Acad Sci U S A. 1989 May;86(9):3065–3069. doi: 10.1073/pnas.86.9.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Mizuuchi K. Retroviral DNA integration: structure of an integration intermediate. Cell. 1988 Aug 12;54(4):497–504. doi: 10.1016/0092-8674(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Hagino-Yamagishi K., Kano K., Mano Y. Effects of aphidicolin on retrovirus DNA synthesis in vivo. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1372–1378. doi: 10.1016/s0006-291x(81)80163-5. [DOI] [PubMed] [Google Scholar]
- Herrick G., Spear B. B., Veomett G. Intracellular localization of mouse DNA polymerase-alpha. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1136–1139. doi: 10.1073/pnas.73.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Hynes R. Six-NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977 Jan;21(1):309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. C., Yang W. K., Tennant R. W., Brown A. Transfection of Fv-1 permissive and restrictive mouse cells with integrated DNA of murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1451–1455. doi: 10.1073/pnas.75.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Taylor J. M. Effect of aphidicolin on avian sarcoma virus replication. J Virol. 1982 Nov;44(2):493–498. doi: 10.1128/jvi.44.2.493-498.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Fate of unintegrated viral DNA in Fv-1 permissive and resistant mouse cells infected with murine leukemia virus. J Virol. 1981 Feb;37(2):609–619. doi: 10.1128/jvi.37.2.609-619.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Rein A., Bassin R. H., Gerwin B. I., Gisselbrecht S. Donation of N- or B-tropic phenotype to NB-tropic murine leukemia virus during mixed infections. J Virol. 1977 Jun;22(3):626–633. doi: 10.1128/jvi.22.3.626-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Merkel G., Kulkosky J., Leis J., Skalka A. M. The avian retroviral IN protein is both necessary and sufficient for integrative recombination in vitro. Cell. 1990 Oct 5;63(1):87–95. doi: 10.1016/0092-8674(90)90290-u. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Lara J., Wambach M. Nontranslated cellular mRNAs are associated with the cytoskeletal framework in influenza virus or adenovirus infected cells. Virology. 1989 Apr;169(2):312–322. doi: 10.1016/0042-6822(89)90156-6. [DOI] [PubMed] [Google Scholar]
- Kozak C. A. Analysis of wild-derived mice for Fv-1 and Fv-2 murine leukemia virus restriction loci: a novel wild mouse Fv-1 allele responsible for lack of host range restriction. J Virol. 1985 Aug;55(2):281–285. doi: 10.1128/jvi.55.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. M., Coffin J. M. Efficient autointegration of avian retrovirus DNA in vitro. J Virol. 1990 Dec;64(12):5958–5965. doi: 10.1128/jvi.64.12.5958-5965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M., Sherr C., Potter M., Todaro G. Isolation of type-C viruses from the Asian feral mouse Mus musculus molossinus. Int J Cancer. 1975 Feb 15;15(2):211–220. doi: 10.1002/ijc.2910150206. [DOI] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Construction of mutants of Moloney murine leukemia virus by suppressor-linker insertional mutagenesis: positions of viable insertion mutations. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4149–4153. doi: 10.1073/pnas.81.13.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel L. I., Murphy J. E., Goff S. P. The palindromic LTR-LTR junction of Moloney murine leukemia virus is not an efficient substrate for proviral integration. J Virol. 1989 Jun;63(6):2629–2637. doi: 10.1128/jvi.63.6.2629-2637.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel L. I., Patel M., King W., Nguyen-Huu M. C., Goff S. P. Construction and recovery of viable retroviral genomes carrying a bacterial suppressor transfer RNA gene. Science. 1985 Apr 19;228(4697):329–332. doi: 10.1126/science.2984770. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Landegent J., Collins C., Fuscoe J., Segraves R., Lucas J., Gray J. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryciak P. M., Sil A., Varmus H. E. Retroviral integration into minichromosomes in vitro. EMBO J. 1992 Jan;11(1):291–303. doi: 10.1002/j.1460-2075.1992.tb05052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. P., Grandgenett D. P. Genetic evidence that the avian retrovirus DNA endonuclease domain of pol is necessary for viral integration. J Virol. 1988 Jul;62(7):2307–2312. doi: 10.1128/jvi.62.7.2307-2312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Kashmiri S. V., Bassin R. H., Gerwin B. L., Duran-Troise G. Phenotypic mixing between N- and B-tropic murine leukemia viruses: infectious particles with dual sensitivity to Fv-1 restriction. Cell. 1976 Mar;7(3):373–379. doi: 10.1016/0092-8674(76)90166-5. [DOI] [PubMed] [Google Scholar]
- Roth M. J., Schwartzberg P. L., Goff S. P. Structure of the termini of DNA intermediates in the integration of retroviral DNA: dependence on IN function and terminal DNA sequence. Cell. 1989 Jul 14;58(1):47–54. doi: 10.1016/0092-8674(89)90401-7. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Construction and analysis of deletion mutations in the pol gene of Moloney murine leukemia virus: a new viral function required for productive infection. Cell. 1984 Jul;37(3):1043–1052. doi: 10.1016/0092-8674(84)90439-2. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Hoffman J., Goff S. P., Baltimore D. Intramolecular integration within Moloney murine leukemia virus DNA. J Virol. 1981 Oct;40(1):164–172. doi: 10.1128/jvi.40.1.164-172.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Soeiro R. Host restriction of Friend leukemia virus: synthesis and integration of the provirus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2356–2360. doi: 10.1073/pnas.73.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek P., Kozak C. HoMuLV: a novel pathogenic ecotropic virus isolated from the European mouse, Mus hortulanus. Virology. 1988 Aug;165(2):469–475. doi: 10.1016/0042-6822(88)90590-9. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Boone L. R., Tennant R. W., Brown A. Restriction of murine leukemia viruses by Fv-1: a model for studying host genetic control of retroviral gene movement and leukemogenesis. Prog Nucleic Acid Res Mol Biol. 1983;29:175–192. doi: 10.1016/s0079-6603(08)60446-8. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. K., Yang D. M., Kiggans J. O., Jr Covalently closed circular DNAs of murine type C retrovirus: depressed formation in cells treated with cycloheximide early after infection. J Virol. 1980 Oct;36(1):181–188. doi: 10.1128/jvi.36.1.181-188.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]