Abstract
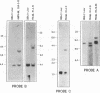
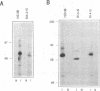
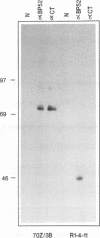
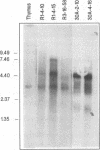
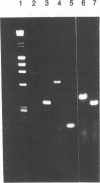
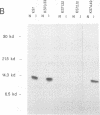
Murine promonocytic leukemias involving insertional mutagenesis of the c-myb locus can be induced by replication-competent retroviruses. In previously studied promonocytic leukemic cells induced by Moloney murine leukemia virus (called MML), the provirus has been invariably integrated upstream of exons 3 or 4 and the leukemic cells expressed aberrant RNAs with fused virus-myb sequences. Furthermore, Myb expressed by these cells has been shown to be truncated by 47 or 71 amino acids. The present report examines the mechanisms of myb activation in leukemias induced by two other retroviruses, amphotropic virus 4070A and Friend strain FB29 (the leukemias are called AMPH-ML and FB-ML, respectively). This study revealed two additional c-myb proviral insertion sites in these promonocytic leukemias. One FB-ML had a proviral integration in exon 9, and expressed a C-terminally truncated Myb protein of 47 kDa similar to that previously demonstrated to be expressed in the myelomonocytic cell lines NFS60 and VFL-2. However, a sequence of reverse-transcribed and amplified RNA from this leukemia demonstrated that the truncation involved a loss of 248 amino acids compared with a loss of 240 amino acids in the myelomonocytic cell lines. Another leukemia had a provirus integrated in the 5' end of c-myb upstream of exon 2 (in the first intron) and produced a Myb protein that was indistinguishable on sodium dodecyl sulfate-polyacrylamide gel electrophoresis from normal Myb. This latter leukemia (FB-ML R1-4-10) expressed Myb with the smallest N-terminal truncation observed so far in promonocytic leukemias; translation begins at an ATG within c-myb exon 2, leading to loss of only 20 amino acids from the N terminus. Unlike the proteins produced in Moloney murine leukemia virus-induced promonocytic leukemias (MML) that have larger truncations, this protein has an intact DNA binding region and does not contain N-terminal amino acids encoded by gag. However, this protein is similar to all N-terminally truncated Mybs so far studied, in that the truncation resulted in deletion of a casein kinase II phosphorylation site which has been proposed to be involved in regulation of DNA binding.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Bender T. P., Kuehl W. M. Murine myb protooncogene mRNA: cDNA sequence and evidence for 5' heterogeneity. Proc Natl Acad Sci U S A. 1986 May;83(10):3204–3208. doi: 10.1073/pnas.83.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Lipsick J. S., Baluda M. A. Antibodies to the evolutionarily conserved amino-terminal region of the v-myb-encoded protein detect the c-myb protein in widely divergent metazoan species. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4685–4689. doi: 10.1073/pnas.83.13.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ferre F., Garduno F. Preparation of crude cell extract suitable for amplification of RNA by the polymerase chain reaction. Nucleic Acids Res. 1989 Mar 11;17(5):2141–2141. doi: 10.1093/nar/17.5.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Cory S., Sobieszczuk P., Holtzman D., Adams J. M. Generation of altered transcripts by retroviral insertion within the c-myb gene in two murine monocytic leukemias. J Virol. 1987 Sep;61(9):2754–2763. doi: 10.1128/jvi.61.9.2754-2763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Ramsay R. G., Johnson G. R. Murine myeloid cell lines derived by in vitro infection with recombinant c-myb retroviruses express myb from rearranged vector proviruses. EMBO J. 1989 Jun;8(6):1767–1775. doi: 10.1002/j.1460-2075.1989.tb03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Kanter M. R., Smith R. E., Hayward W. S. Rapid induction of B-cell lymphomas: insertional activation of c-myb by avian leukosis virus. J Virol. 1988 Apr;62(4):1423–1432. doi: 10.1128/jvi.62.4.1423-1432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavu S., Reddy E. P. Structural organization and nucleotide sequence of mouse c-myb oncogene: activation in ABPL tumors is due to viral integration in an intron which results in the deletion of the 5' coding sequences. Nucleic Acids Res. 1986 Jul 11;14(13):5309–5320. doi: 10.1093/nar/14.13.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part II. Myb. Genes Dev. 1990 Dec;4(12B):2235–2241. doi: 10.1101/gad.4.12b.2235. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., McKinney M. D., Agranovsky O. Contribution of the gag and pol sequences to the leukemogenicity of Friend murine leukemia virus. J Virol. 1985 Jun;54(3):864–868. doi: 10.1128/jvi.54.3.864-868.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Perryman S., Nishio J., Chesebro B. Complete nucleotide sequence of Friend murine leukemia virus, strain FB29. Nucleic Acids Res. 1991 Dec 25;19(24):6950–6950. doi: 10.1093/nar/19.24.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer E. S., Baba T. W., Humphries E. H. Activation of the c-myb locus is insufficient for the rapid induction of disseminated avian B-cell lymphoma. J Virol. 1992 Jan;66(1):512–523. doi: 10.1128/jvi.66.1.512-523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer E., Humphries E. H. RAV-1 insertional mutagenesis: disruption of the c-myb locus and development of avian B-cell lymphomas. J Virol. 1989 Apr;63(4):1630–1640. doi: 10.1128/jvi.63.4.1630-1640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., De Billy G., Wang P., Darlix J. L. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J Mol Biol. 1989 Jan 20;205(2):363–372. doi: 10.1016/0022-2836(89)90347-1. [DOI] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. G., Ishii S., Nishina Y., Soe G., Gonda T. J. Characterization of alternate and truncated forms of murine c-myb proteins. Oncogene Res. 1989;4(4):259–269. [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Saris C. J., Domen J., Berns A. The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J. 1991 Mar;10(3):655–664. doi: 10.1002/j.1460-2075.1991.tb07994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Wolff L. Moloney murine leukemia virus-induced myeloid tumors in adult BALB/c mice: requirement of c-myb activation but lack of v-abl involvement. J Virol. 1987 Dec;61(12):3721–3725. doi: 10.1128/jvi.61.12.3721-3725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Sitbon M., Nishio J., Wehrly K., Lodmell D., Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985 Feb;141(1):110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Cleveland J. L., Askew D. S., Rapp U. R., Ihle J. N. Insertion and truncation of c-myb by murine leukemia virus in a myeloid cell line derived from cultures of normal hematopoietic cells. J Virol. 1987 Jul;61(7):2339–2343. doi: 10.1128/jvi.61.7.2339-2343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Koller R., Davidson W. Acute myeloid leukemia induction by amphotropic murine retrovirus (4070A): clonal integrations involve c-myb in some but not all leukemias. J Virol. 1991 Jul;65(7):3607–3616. doi: 10.1128/jvi.65.7.3607-3616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Koller R. Regions of the Moloney murine leukemia virus genome specifically related to induction of promonocytic tumors. J Virol. 1990 Jan;64(1):155–160. doi: 10.1128/jvi.64.1.155-160.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Mushinski J. F., Shen-Ong G. L., Morse H. C., 3rd A chronic inflammatory response. Its role in supporting the development of c-myb and c-myc related promonocytic and monocytic tumors in BALB/c mice. J Immunol. 1988 Jul 15;141(2):681–689. [PubMed] [Google Scholar]