Abstract
Naturally occurring recombinant murine leukemia viruses (MuLVs), termed mink cell focus-inducing (MCF) viruses, are the proximal leukemogens in spontaneous thymic lymphomas of AKR mice. The mechanism by which these viruses transform lymphocytes is not clear. Previous studies have implicated either integrational activation of proto-oncogenes, chronic autocrine immune stimulation, and/or autocrine stimulation of growth factor receptors (e.g., interleukin 2 receptors) via binding of the viral env glycoprotein (gp70) to these receptors. Any one of these events could also involve activation of second messenger signaling pathways in the cell. We examined whether infection with oncogenic AKR-247 MCF MuLV induced transmembrane signaling cascades in thymocytes of AKR mice. Cyclic AMP levels were not changed, but there was enhanced turnover of phosphatidylinositol phosphates, with concomitant increases in diacyglycerol and inositol 1,4,5-triphosphate. Thus, phospholipase C activity was increased. Protein kinase C activity was also elevated in comparison to that in uninfected thymocytes. The above events occurred in parallel with MCF expression in the thymus and were chronically maintained thereafter. No changes in phospholipid turnover occurred in an organ which did not replicate the MCF virus (spleen) or in thymocytes of AKR mice infected with a thymotropic, nononcogenic MCF virus (AKV-1-C36). Therefore, only the oncogenic MCF virus induced phosphatidylinositol signal transduction. Flow cytometric comparison of cell surface gp70 revealed that AKR-247 MCF virus-infected thymocytes expressed more MCF virus gp70 than did thymocytes from AKV-1-C36 MCF virus-infected mice, suggesting that certain threshold quantities of MCF virus env glycoproteins may be involved in this signaling. This type of signal transduction is not induced by stimulation of the interleukin 2 receptor but is involved in certain oncogene systems (e.g., ras and fms). Its chronic induction by oncogenic MCF MuLV may thus initiate thymocyte transformation.
Full text
PDF

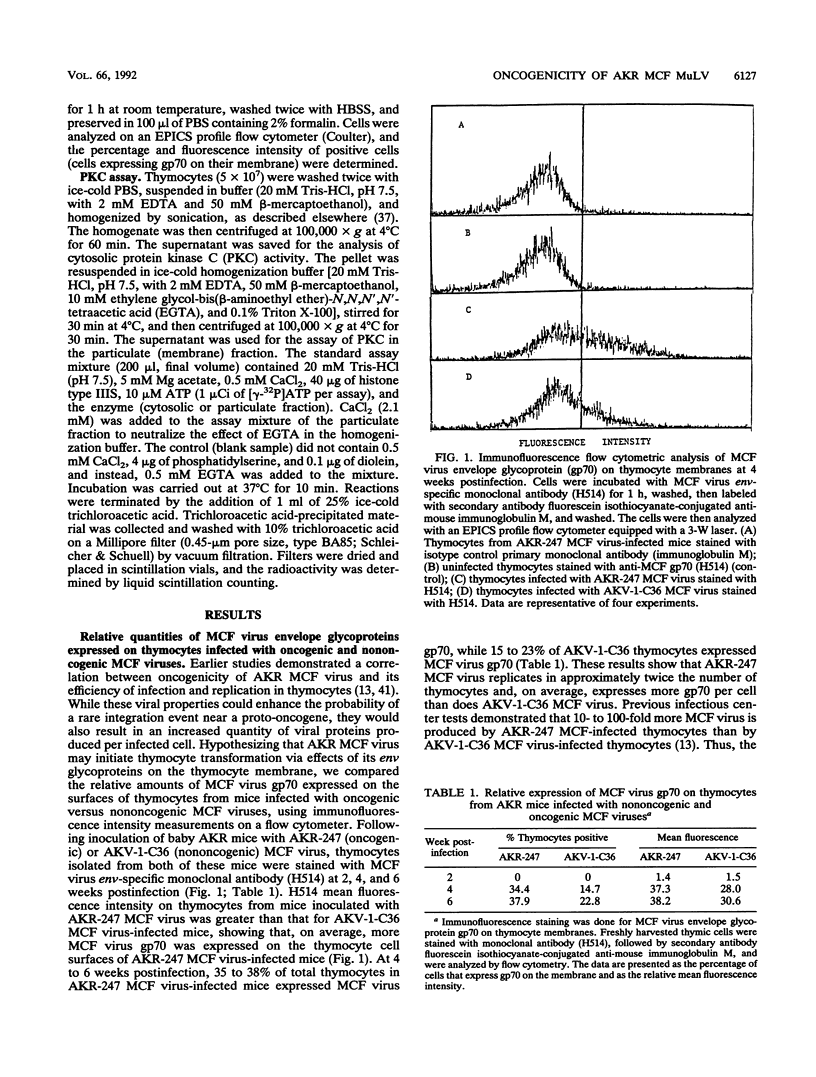
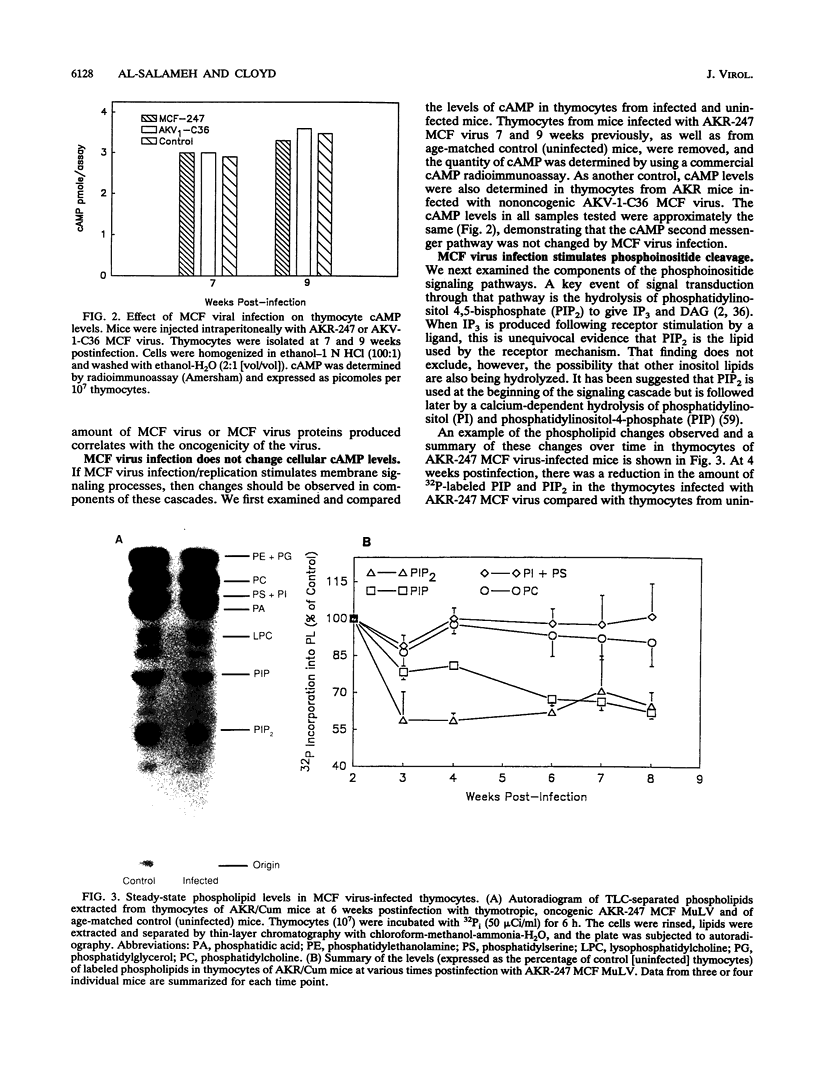
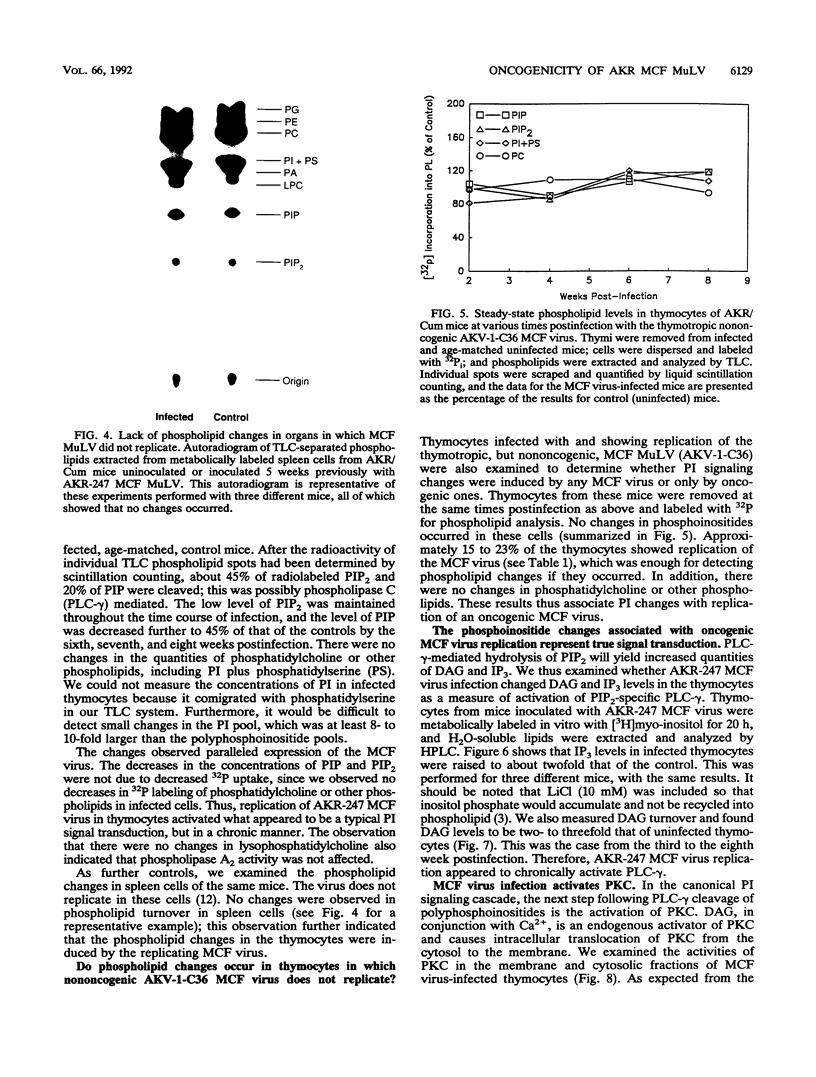


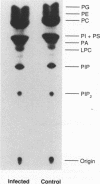
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Bull P., Hunter T., Verma I. M. Transcriptional induction of the murine c-rel gene with serum and phorbol-12-myristate-13-acetate in fibroblasts. Mol Cell Biol. 1989 Nov;9(11):5239–5243. doi: 10.1128/mcb.9.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Chung S. W., Wolff L., Ruscetti S. K. Transmembrane domain of the envelope gene of a polycythemia-inducing retrovirus determines erythropoietin-independent growth. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7957–7960. doi: 10.1073/pnas.86.20.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W. Characterization of target cells for MCF viruses in AKR mice. Cell. 1983 Jan;32(1):217–225. doi: 10.1016/0092-8674(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R. Experimental models and biological mechanisms for tumor promotion. Cancer Cells. 1990 Jan;2(1):8–15. [PubMed] [Google Scholar]
- Farese R. V., Davis J. S., Barnes D. E., Standaert M. L., Babischkin J. S., Hock R., Rosic N. K., Pollet R. J. The de novo phospholipid effect of insulin is associated with increases in diacylglycerol, but not inositol phosphates or cytosolic Ca2+. Biochem J. 1985 Oct 15;231(2):269–278. doi: 10.1042/bj2310269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Chida K., Kamata N., Nose K., Kato M., Homma Y., Takenawa T., Kuroki T. Enhancement of inositol phospholipid metabolism and activation of protein kinase C in ras-transformed rat fibroblasts. J Biol Chem. 1988 Dec 5;263(34):17975–17980. [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Sherr C. J., Rock C. O. A guanine nucleotide-dependent phosphatidylinositol 4,5-diphosphate phospholipase C in cells transformed by the v-fms and v-fes oncogenes. J Biol Chem. 1986 Apr 15;261(11):4978–4985. [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Kato M., Kawai S., Takenawa T. Altered signal transduction in erbB-transformed cells. Implication of enhanced inositol phospholipid metabolism in erbB-induced transformation. J Biol Chem. 1987 Apr 25;262(12):5696–5704. [PubMed] [Google Scholar]
- Lacal J. C., Fleming T. P., Warren B. S., Blumberg P. M., Aaronson S. A. Involvement of functional protein kinase C in the mitogenic response to the H-ras oncogene product. Mol Cell Biol. 1987 Nov;7(11):4146–4149. doi: 10.1128/mcb.7.11.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Ihle J. N. Mechanisms of C-type viral leukemogenesis. I. Correlation of in vitro lymphocyte blastogenesis to viremia and leukemia. J Immunol. 1979 Nov;123(5):2351–2358. [PubMed] [Google Scholar]
- Li J. P., Baltimore D. Mechanism of leukemogenesis induced by mink cell focus-forming murine leukemia viruses. J Virol. 1991 May;65(5):2408–2414. doi: 10.1128/jvi.65.5.2408-2414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Holland C. A., Hartley J. W., Hopkins N. Viral integration near c-myc in 10-20% of mcf 247-induced AKR lymphomas. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6808–6811. doi: 10.1073/pnas.81.21.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Kabat D. Reduced leukemogenicity caused by mutations in the membrane glycoprotein gene of Rauscher spleen focus-forming virus. J Virol. 1984 Feb;49(2):394–402. doi: 10.1128/jvi.49.2.394-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Neufeld E. J., Wilson D. B. Production of phosphoinositide-derived messengers. Cell. 1984 Jul;37(3):701–703. doi: 10.1016/0092-8674(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Malhotra R. K., Bhave S. V., Wakade T. D., Wakade A. R. Protein kinase C of sympathetic neuronal membrane is activated by phorbol ester--correlation between transmitter release, 45Ca2+ uptake, and the enzyme activity. J Neurochem. 1988 Sep;51(3):967–974. doi: 10.1111/j.1471-4159.1988.tb01834.x. [DOI] [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Fleissner E., Lonial H., Koehne C. F., Reicin A. Early clonality and high-frequency proviral integration into the c-myc locus in AKR leukemias. J Virol. 1985 Aug;55(2):500–503. doi: 10.1128/jvi.55.2.500-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E., Obata Y., Old L. J. Leukemogenic properties of AKR dualtropic (MCF) viruses: amplification of murine leukemia virus-related antigens on thymocytes and acceleration of leukemia development in AKR mice. Virology. 1981 Jul 30;112(2):548–563. doi: 10.1016/0042-6822(81)90301-9. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Traganos F. Changes in thymocyte proliferation at different stages of viral leukemogenesis in AKR mice. J Immunol. 1986 Jan;136(2):720–727. [PubMed] [Google Scholar]
- O'Donnell P. V., Woller R., Chu A. Stages in development of mink cell focus-inducing (MCF) virus-accelerated leukemia in AKR mice. J Exp Med. 1984 Sep 1;160(3):914–934. doi: 10.1084/jem.160.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicin A., Yang J. Q., Marcu K. B., Fleissner E., Koehne C. F., O'Donnell P. V. Deregulation of the c-myc oncogene in virus-induced thymic lymphomas of AKR/J mice. Mol Cell Biol. 1986 Nov;6(11):4088–4092. doi: 10.1128/mcb.6.11.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., Denny C. T., Witte O. N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991 Jan 25;64(2):337–350. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Zijlstra M., Melief C., Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984 Dec 20;3(13):3215–3222. doi: 10.1002/j.1460-2075.1984.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey N. A., Leach K. L., Blumberg P. M. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci U S A. 1984 Jan;81(2):607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Steffen D. Proviruses are adjacent to c-myc in some murine leukemia virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2097–2101. doi: 10.1073/pnas.81.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Sukumar S. ras oncogenes in chemical carcinogenesis. Curr Top Microbiol Immunol. 1989;148:93–114. doi: 10.1007/978-3-642-74700-7_3. [DOI] [PubMed] [Google Scholar]
- Thomassen D. G., Gilmer T. M., Annab L. A., Barrett J. C. Evidence for multiple steps in neoplastic transformation of normal and preneoplastic Syrian hamster embryo cells following transfection with Harvey murine sarcoma virus oncogene (v-Ha-ras). Cancer Res. 1985 Feb;45(2):726–732. [PubMed] [Google Scholar]
- Travali S., Reiss K., Ferber A., Petralia S., Mercer W. E., Calabretta B., Baserga R. Constitutively expressed c-myb abrogates the requirement for insulinlike growth factor 1 in 3T3 fibroblasts. Mol Cell Biol. 1991 Feb;11(2):731–736. doi: 10.1128/mcb.11.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Bear S. E. Infection by mink cell focus-forming viruses confers interleukin 2 (IL-2) independence to an IL-2-dependent rat T-cell lymphoma line. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4611–4615. doi: 10.1073/pnas.88.11.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Neufeld E. J., Majerus P. W. Phosphoinositide interconversion in thrombin-stimulated human platelets. J Biol Chem. 1985 Jan 25;260(2):1046–1051. [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985 Jun 28;228(4707):1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]