Abstract
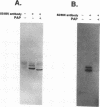
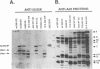
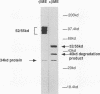
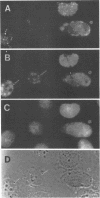
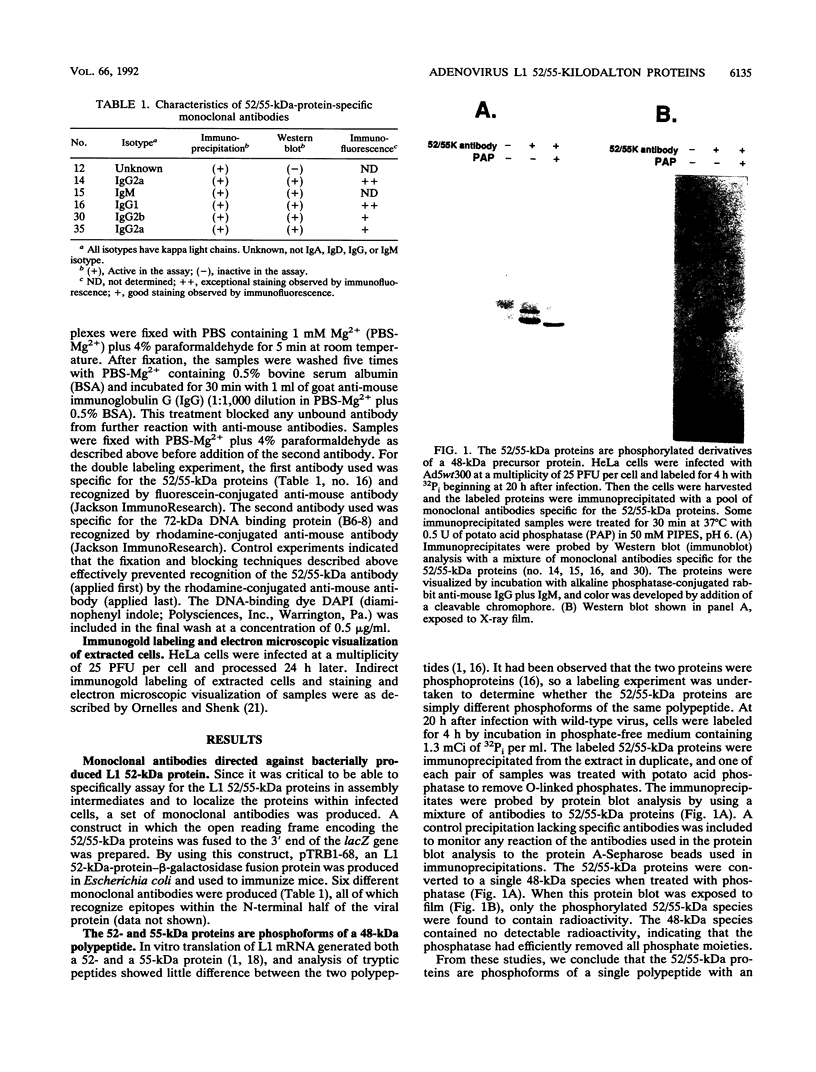
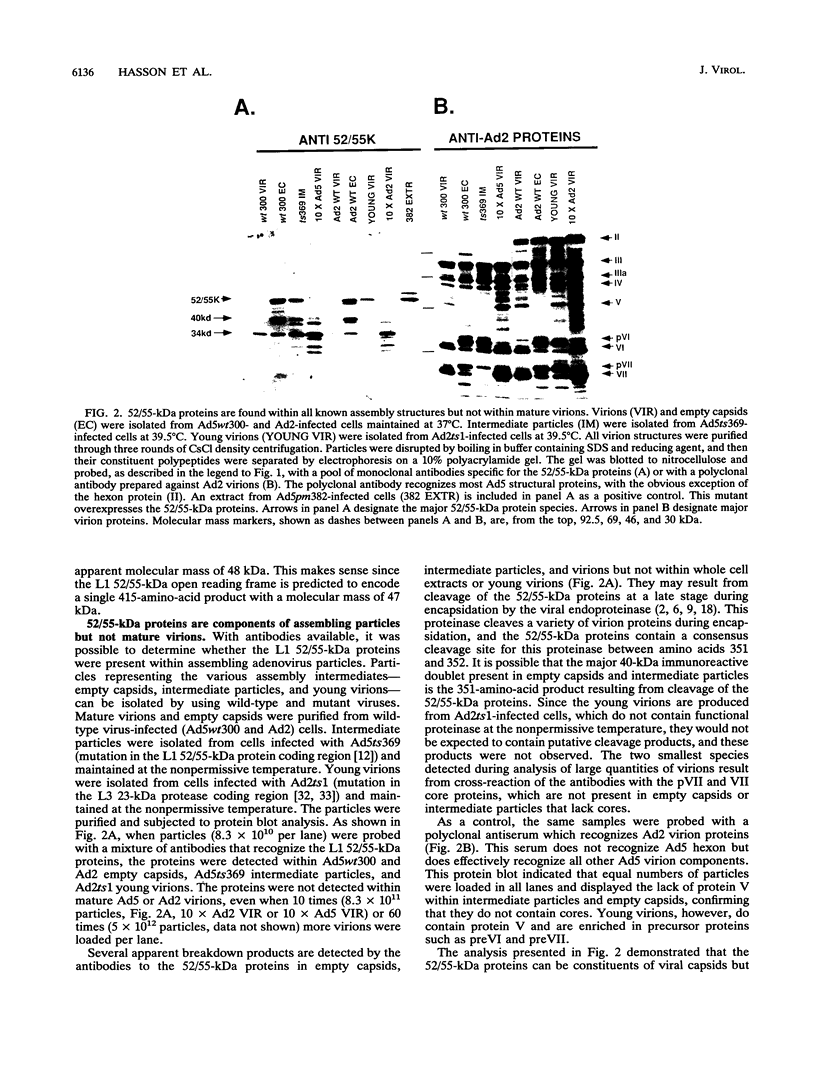
Analysis of a temperature-sensitive mutant, Ad5ts369, had indicated that the adenovirus L1 52- and 55-kDa proteins (52/55-kDa proteins) are required for the assembly of infectious virions. By using monoclonal antibodies directed against bacterially produced L1 52-kDa protein, the L1 52/55-kDa proteins were found to be differentially phosphorylated forms of a single 48-kDa polypeptide. Both phosphoforms were shown to be present within all suspected virus assembly intermediates (empty capsids, 50 to 100 molecules; young virions, 1 to 2 molecules) but not within mature virions. The mobilities of these proteins in polyacrylamide gels were affected by reducing agents, indicating that the 52/55-kDa proteins may exist as homodimers within the cell and within assembling particles. Immunofluorescence analysis revealed that the 52/55-kDa proteins localize to regions within the infected nucleus that are distinct from viral DNA replication centers, indicating that replication and assembly of viral components likely occur in separate nuclear compartments. Immunoelectron microscopic studies determined that the 52/55-kDa proteins are found in close association with structures that appear to contain assembling virions. These results are consistent with an active but transient role for the L1 products in assembly of the adenovirus particle, perhaps as scaffolding proteins.
Full text
PDF


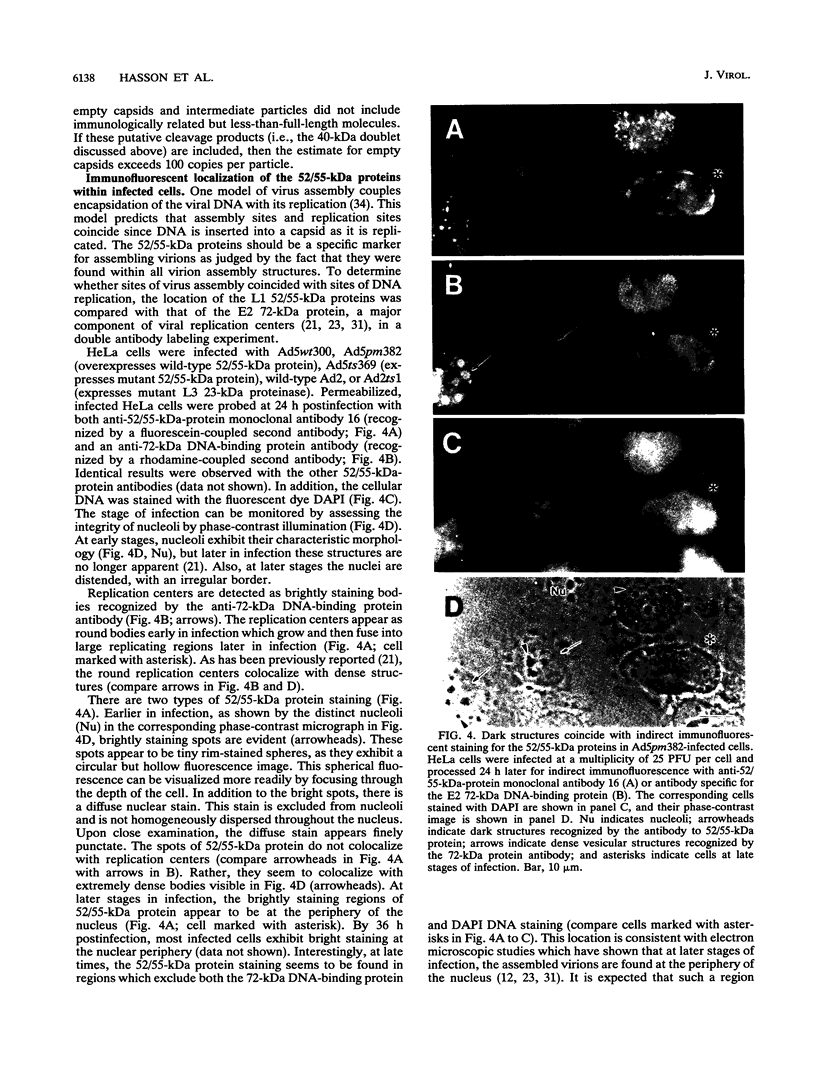

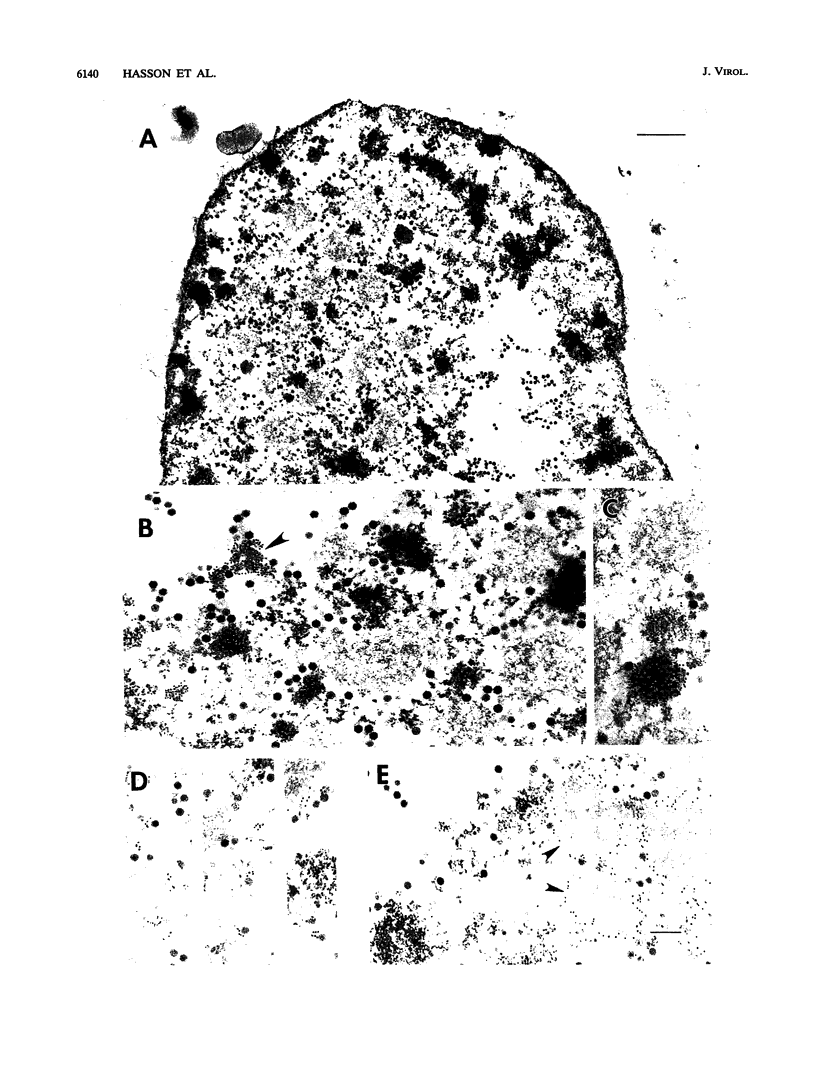


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin M. L., D'Halluin J. C., Cousin C., Boulanger P. Human adenovirus type 2 protein IIIa. II. Maturation and encapsidation. Virology. 1980 Feb;101(1):144–156. doi: 10.1016/0042-6822(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Chroboczek J., Viard F., D'Halluin J. C. Human adenovirus 2 temperature-sensitive mutant 112 contains three mutations in the protein IIIa gene. Gene. 1986;49(1):157–160. doi: 10.1016/0378-1119(86)90396-3. [DOI] [PubMed] [Google Scholar]
- D'Halluin J. C., Martin G. R., Torpier G., Boulanger P. A. Adenovirus type 2 assembly analyzed by reversible cross-linking of labile intermediates. J Virol. 1978 May;26(2):357–363. doi: 10.1128/jvi.26.2.357-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. A., Martin G. R. Temperature-sensitive mutant of adenovirus type 2 blocked in virion assembly: accumulation of light intermediate particles. J Virol. 1978 May;26(2):344–356. doi: 10.1128/jvi.26.2.344-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980 Jul;20(3):787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- Hasson T. B., Soloway P. D., Ornelles D. A., Doerfler W., Shenk T. Adenovirus L1 52- and 55-kilodalton proteins are required for assembly of virions. J Virol. 1989 Sep;63(9):3612–3621. doi: 10.1128/jvi.63.9.3612-3621.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Fahnestock M. L., Hardy M. M., Anderson C. W. Presence in infected cells of nonvirion proteins encoded by adenovirus messenger RNAs of the major late transcription regions L0 and L1. Virology. 1985 Jun;143(2):452–466. doi: 10.1016/0042-6822(85)90385-x. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Symington J. S., Green M. Biosynthesis and properties of the adenovirus 2 L1-encoded 52,000- and 55,000-Mr proteins. J Virol. 1986 Mar;57(3):839–847. doi: 10.1128/jvi.57.3.839-847.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Granboulan N. Electron microscopy of adenovirus 12 replication. II. High-resolution autoradiography of infected KB cells labeled with tritiated thymidine. J Virol. 1967 Oct;1(5):1010–1018. doi: 10.1128/jvi.1.5.1010-1018.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. S., Ricciardi R. P., Roberts B. E., Paterson B. M., Mathews M. B. Arrangement of messenger RNAs and protein coding sequences in the major late transcription unit of adenovirus 2. J Mol Biol. 1980 Oct 5;142(4):455–488. doi: 10.1016/0022-2836(80)90258-2. [DOI] [PubMed] [Google Scholar]
- Moyne G., Pichard E., Bernhard W. Localization of simian adenovirus 7 (SA 7) transcription and replication in lytic infection. An ultracytochemical and autoradiographical study. J Gen Virol. 1978 Jul;40(1):77–92. doi: 10.1099/0022-1317-40-1-77. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Ornelles D. A., Shenk T. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991 Jan;65(1):424–429. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Pédron J., Cajean-Feroldi C. Identification of intranuclear structures containing the 72K DNA-binding protein of human adenovirus type 5. Eur J Cell Biol. 1984 Jul;34(2):313–322. [PubMed] [Google Scholar]
- Reich N. C., Sarnow P., Duprey E., Levine A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983 Jul 30;128(2):480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- Rosenwirth B., Tjia S., Westphal M., Doerfler W. Incomplete particles of adenovirus. II. Kinetics of formation and polypeptide composition of adenovirus type 2. Virology. 1974 Aug;60(2):431–437. doi: 10.1016/0042-6822(74)90337-7. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Yamaguchi K. The viral DNA replication complex of adenovirus 12. Virology. 1974 Jul;60(1):192–199. doi: 10.1016/0042-6822(74)90376-6. [DOI] [PubMed] [Google Scholar]
- Simmons T., Heywood P., Hodge L. D. Intranuclear site of replication of adenovirus DNA. J Mol Biol. 1974 Nov 5;89(3):423–433. doi: 10.1016/0022-2836(74)90473-2. [DOI] [PubMed] [Google Scholar]
- Soloway P. D., Shenk T. The adenovirus type 5 i-leader open reading frame functions in cis to reduce the half-life of L1 mRNAs. J Virol. 1990 Feb;64(2):551–558. doi: 10.1128/jvi.64.2.551-558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Viral DNA sequences from incomplete particles of human adenovirus type 7. Cell. 1977 Sep;12(1):243–249. doi: 10.1016/0092-8674(77)90202-1. [DOI] [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. M., Déry C. V., Mirza M. A., Horvath J. Adenovirus DNA synthesis is coupled to virus assembly. Virology. 1985 Jan 30;140(2):351–359. doi: 10.1016/0042-6822(85)90371-x. [DOI] [PubMed] [Google Scholar]
- Weber J., Begin M., Khittoo G. Genetic Analysis of Adenovirus Type 2 II. Preliminary Phenotypic Characterization of Temperature-Sensitive Mutants. J Virol. 1975 May;15(5):1049–1056. doi: 10.1128/jvi.15.5.1049-1056.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]