Abstract
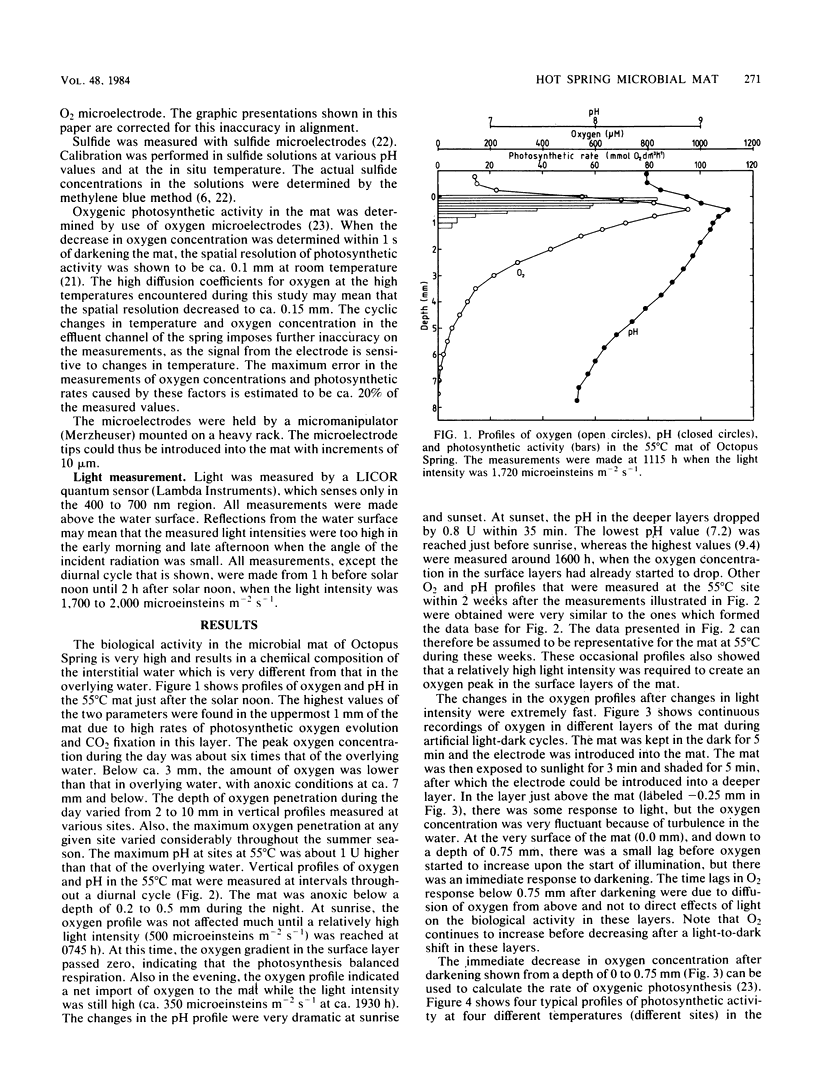
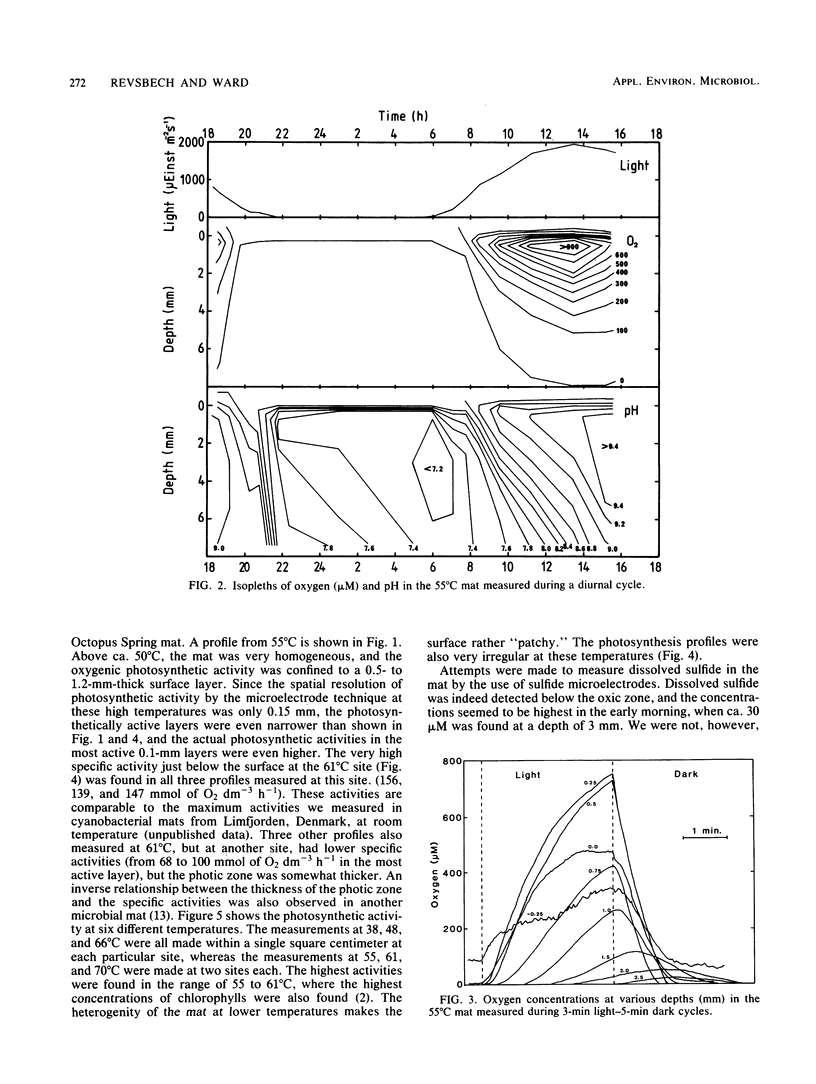
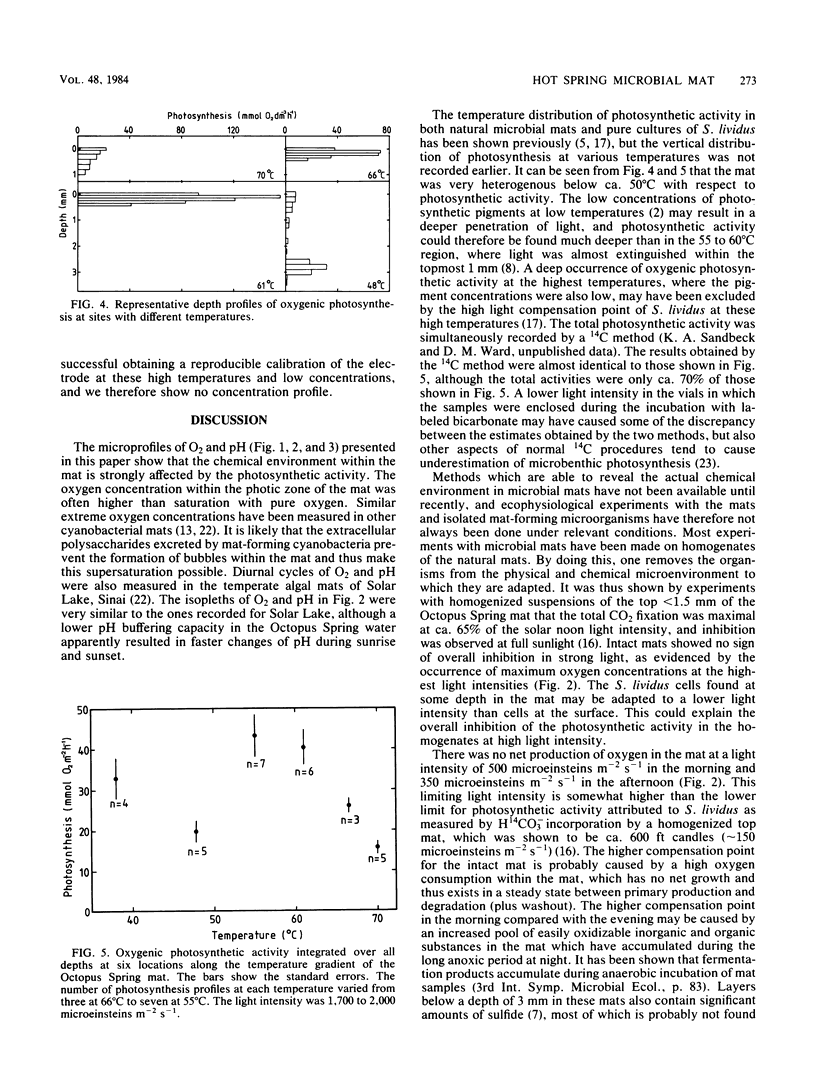
Microelectrodes were used to measure oxygen, pH, and oxygenic photosynthetic activity in a hot spring microbial mat (Octopus Spring, Yellowstone National Park), where the cyanobacterium Synechococcus lividus and the filamentous bacterium Chloroflexus aurantiacus are the only known phototrophs. The data showed very high biological activities in the topmost layers of the microbial mat, resulting in extreme values for oxygen and pH. At a 1-mm depth at a 55°C site, oxygen and pH reached 900 μM and 9.4, respectively, just after solar noon, whereas anoxic conditions with a pH of 7.2 were measured before sunrise. Although diurnal changes between these extremes occurred over hours during a diurnal cycle, microbial activity was great enough to give the same response in 1 to 2 min after artificial shading. Oxygenic photosynthesis was confined to a 0.5- to 1.1-mm layer at sites with temperatures at or above about 50°C, with maximum activities in the 55 to 60°C region. The data suggest that S. lividus is the dominant primary producer of the mat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doemel W. N., Brock T. D. Structure, growth, and decomposition of laminated algal-bacterial mats in alkaline hot springs. Appl Environ Microbiol. 1977 Oct;34(4):433–452. doi: 10.1128/aem.34.4.433-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen B. B., Revsbech N. P., Blackburn T. H., Cohen Y. Diurnal cycle of oxygen and sulfide microgradients and microbial photosynthesis in a cyanobacterial mat sediment. Appl Environ Microbiol. 1979 Jul;38(1):46–58. doi: 10.1128/aem.38.1.46-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Brock T. D. Adaptation by hot spring phototrophs to reduced light intensities. Arch Microbiol. 1977 May 13;113(1-2):111–120. doi: 10.1007/BF00428590. [DOI] [PubMed] [Google Scholar]
- Madigan M. T., Brock T. D. Photosynthetic sulfide oxidation by Chloroflexus aurantiacus, a filamentous, photosynthetic, gliding bacterium. J Bacteriol. 1975 May;122(2):782–784. doi: 10.1128/jb.122.2.782-784.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Castenholz R. W. Growth and photosynthesis in an extreme thermophile, Synechococcus lividus (Cyanophyta). Arch Mikrobiol. 1971;78(1):25–41. doi: 10.1007/BF00409086. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- Sandbeck K. A., Ward D. M. Fate of immediate methane precursors in low-sulfate, hot-spring algal-bacterial mats. Appl Environ Microbiol. 1981 Mar;41(3):775–782. doi: 10.1128/aem.41.3.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., Fuller R. C. Semiaerobic induction of bacteriochlorophyll synthesis in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1032–1039. doi: 10.1128/jb.147.3.1032-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M., Olson G. J. Terminal processes in the anaerobic degradation of an algal-bacterial mat in a high-sulfate hot spring. Appl Environ Microbiol. 1980 Jul;40(1):67–74. doi: 10.1128/aem.40.1.67-74.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M. Thermophilic methanogenesis in a hot-spring algal-bacterial mat (71 to 30 degrees C). Appl Environ Microbiol. 1978 Jun;35(6):1019–1026. doi: 10.1128/aem.35.6.1019-1026.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



