Abstract
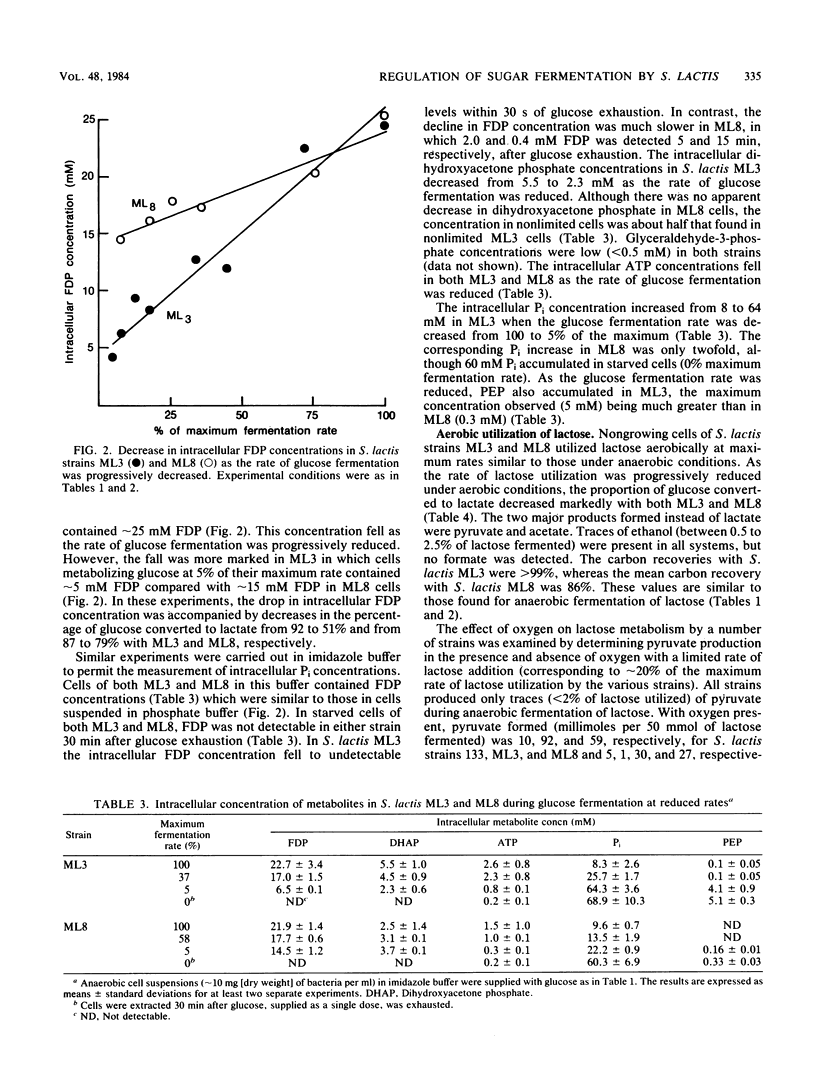
Nongrowing cells of Streptococcus lactis in a pH-stat were dosed with sugar to allow fermentation at the maximum rate or were fed a continuous supply of sugar at rates less than the maximum. Under anaerobic conditions, rapid fermentation of either glucose or lactose was essentially homolactic. However, with strain ML3, limiting the fermentation rate diverted approximately half of the pyruvate to formate, acetate, and ethanol. At limiting glucose fermentation rates, cells contained lower concentrations of lactate dehydrogenase activator (fructose 1,6-diphosphate) and pyruvate formate-lyase inhibitors (triose phosphates). As a result, pyruvate formate-lyase and pyruvate dehydrogenase play a greater role in pyruvate metabolism. In contrast to strain ML3, strain ML8 did not give the same diversion of products under anaerobic conditions, and cells retained higher concentrations of the above effector compounds. Lactose metabolism under aerobic conditions resulted in pyruvate excretion by both S. lactis ML3 and ML8. At 7% of the maximum utilization rate, pyruvate accounted for 69 and 35% of the lactose metabolized by ML3 and ML8, respectively. Acetate was also a major product, especially with ML8. The data suggest that NADH oxidase is involved in coenzyme recycling in the presence of oxygen and that pyruvate formate-lyase is inactivated, but the pyruvate dehydrogenase complex still functions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Involvement of oxygen-sensitive pyruvate formate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982 Oct;152(1):175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders R. F., Hogg D. M., Jago G. R. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl Microbiol. 1970 Apr;19(4):608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome M. C., Thomas M. P., Hillier A. J., Jago G. R. Pyruvate dehydrogenase activity in group N streptococci. Aust J Biol Sci. 1980 Mar;33(1):15–25. [PubMed] [Google Scholar]
- Chen J. H., Jones R. F. Multiple forms of phosphoenolpyruvate carboxylase from Chlamydomonas reeinhardtii. Biochim Biophys Acta. 1970 Aug 21;214(2):318–325. doi: 10.1016/0005-2795(70)90009-7. [DOI] [PubMed] [Google Scholar]
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell N. W., Veech R. L. Enzymatic measurement of ethanol or NAD in acid extracts of biological samples. Anal Biochem. 1983 Jul 15;132(2):418–423. doi: 10.1016/0003-2697(83)90029-5. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Purification and properties of pyruvate kinase from Streptococcus lactis. Biochim Biophys Acta. 1976 Jun 7;438(1):90–101. doi: 10.1016/0005-2744(76)90225-4. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Thomas T. D. D-tagatose 1,6-diphosphate aldolase from lactic streptococci: purification, properties, and use in measuring intracellular tagatose 1,6-diphosphate. J Bacteriol. 1982 Aug;151(2):600–608. doi: 10.1128/jb.151.2.600-608.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demko G. M., Blanton S. J., Benoit R. E. Heterofermentative carbohydrate metabolism of lactose-impaired mutants of Streptococcus lactis. J Bacteriol. 1972 Dec;112(3):1335–1345. doi: 10.1128/jb.112.3.1335-1345.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow J. A. Lactose hydrolysing enzymes in Streptococcus lactis and Streptococcus cremoris and also in some other species of streptococci. J Appl Bacteriol. 1980 Dec;49(3):493–503. doi: 10.1111/j.1365-2672.1980.tb04724.x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Altered metabolism in a Streptococcus lactis C2 mutant deficient in lactic dehydrogenase. J Dairy Sci. 1974 Feb;57(2):181–186. doi: 10.3168/jds.S0022-0302(74)84857-5. [DOI] [PubMed] [Google Scholar]
- Mou L., Mulvena D. P., Jonas H. A., Jago G. R. Purification and properties of nicotinamide adenine dinucleotide-dependent D- and L- lactate dehydrogenases in a group N streptococcus. J Bacteriol. 1972 Aug;111(2):392–396. doi: 10.1128/jb.111.2.392-396.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLATT T. B., FOSTER E. M. Products of glucose metabolism by homofermentative streptococci under anaerobic conditions. J Bacteriol. 1958 Apr;75(4):453–459. doi: 10.1128/jb.75.4.453-459.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal B. D., Maeba P. Phosphoenolpyruvate carboxylase: activation by nucleotides as a possible compensatory feedback effect. J Biol Chem. 1966 Oct 10;241(19):4557–4562. [PubMed] [Google Scholar]
- Schulz D. W., Passonneau J. V., Lowry O. H. An enzymic method for the measurement of inorganic phosphate. Anal Biochem. 1967 May;19(2):300–314. doi: 10.1016/0003-2697(67)90166-2. [DOI] [PubMed] [Google Scholar]
- Stowell A. R., Crow K. E., Greenway R. M., Batt R. D. Determination of acetaldehyde in blood using automated distillation and fluorometry. Anal Biochem. 1978 Feb;84(2):384–392. doi: 10.1016/0003-2697(78)90055-6. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Jarvis B. D., Skipper N. A. Localization of proteinase(s) near the cell surface of Streptococcus lactis. J Bacteriol. 1974 May;118(2):329–333. doi: 10.1128/jb.118.2.329-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Tagatose-1, 6-diphosphate activation of lactate dehydrogenase from Streptococcus cremoris. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1035–1042. doi: 10.1016/0006-291x(75)90673-7. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W., Crow V. L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980 Nov;144(2):672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]


