Abstract
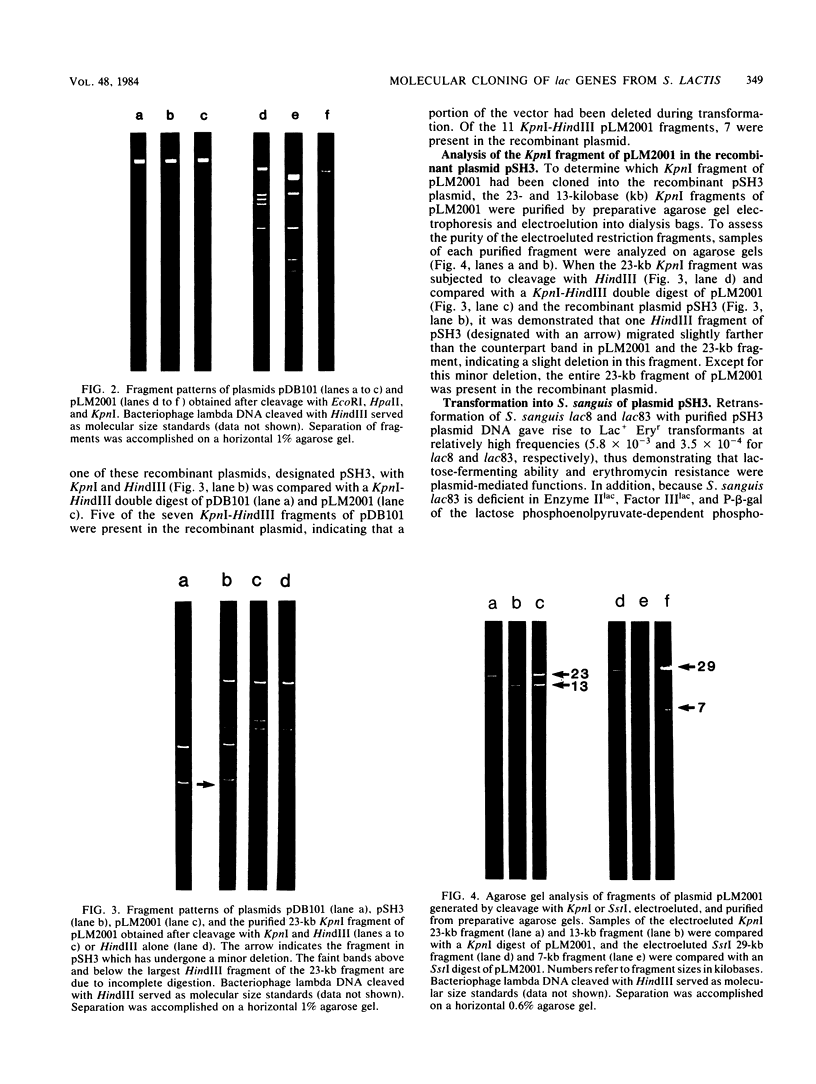
Restriction endonucleases and agarose gel electrophoresis were used to analyze plasmid pLM2001, which is required for lactose metabolism by Streptococcus lactis LM0232. The enzymes XhoI, SstI, BamHI, and KpnI each cleaved the plasmid into two fragments, whereas EcoRI and BglII cleaved the plasmid into seven and five fragments, respectively. Sizing of fragments and multiple digestions allowed construction of a composite restriction map. The KpnI fragments of pLM2001 were cloned into the KpnI cleavage site of the vector plasmid pDB101. A recombinant plasmid (pSH3) obtained from a lactose-fermenting, erythromycin-resistant (Lac+ Eryr) transformant of Streptococcus sanguis Challis was analyzed by enzyme digestion and agarose gel electrophoresis. Plasmid pSH3 contained 7 of the 11 KpnI-HindIII fragments from pLM2001 and 5 of the 7 fragments from pDB101. It was determined that a 23-kilobase (kb) KpnI-generated fragment from pLM2001 had been cloned into pDB101 with deletion of part of the vector plasmid. The recombinant plasmid could be transformed with high frequency into several Lac- strains of S. sanguis, conferring the ability to ferment lactose and erythromycin resistance. The presence of pSH3 allowed a strain deficient in Enzyme IIlac, Factor IIIlac, and phospho-beta-galactosidase of the lactose phosphoenolpyruvate-dependent phosphotransferase system to efficiently ferment lactose. Under conditions designed to maximize curing of plasmid DNA with acriflavin, no Lac- derivatives could be isolated from cells transformed with pSH3. Seven of the 40 Lac+ colonies isolated after 10 transfers in acriflavin were shown to be sensitive to erythromycin and did not appear to harbor plasmid DNA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Genetic and physical characterization of recombinant plasmids associated with cell aggregation and high-frequency conjugal transfer in Streptococcus lactis ML3. J Bacteriol. 1984 Jun;158(3):954–962. doi: 10.1128/jb.158.3.954-962.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Ferretti J. J. Physical mapping of plasmid pDB101: a potential vector plasmid for molecular cloning in streptococci. Plasmid. 1980 Sep;4(2):130–138. doi: 10.1016/0147-619x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Behnke D., Gilmore M. S., Ferretti J. J. Plasmid pGB301, a new multiple resistance streptococcal cloning vehicle and its use in cloning of a gentamicin/kanamycin resistance determinant. Mol Gen Genet. 1981;182(3):414–421. doi: 10.1007/BF00293929. [DOI] [PubMed] [Google Scholar]
- Behnke D., Malke H., Hartmann M., Walter F. Post-transformational rearrangement of an in vitro reconstructed group-A streptococcal erythromycin resistance plasmid. Plasmid. 1979 Oct;2(4):605–616. doi: 10.1016/0147-619x(79)90058-1. [DOI] [PubMed] [Google Scholar]
- Behnke D. Plasmid transformation of Streptococcus sanguis (Challis) occurs by circular and linear molecules. Mol Gen Genet. 1981;182(3):490–497. doi: 10.1007/BF00293940. [DOI] [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1980 Nov;18(5):753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Ghosal D., Saedler H. Tn951: a new transposon carrying a lactose operon. Mol Gen Genet. 1978 Apr 6;160(2):215–224. doi: 10.1007/BF00267484. [DOI] [PubMed] [Google Scholar]
- Harlander S. K., McKay L. L. Transformation of Streptococcus sanguis Challis with Streptococcus lactis plasmid DNA. Appl Environ Microbiol. 1984 Aug;48(2):342–346. doi: 10.1128/aem.48.2.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Larsen L. D., McKay L. L. Plasmid Profiles of Lactose-Negative and Proteinase-Deficient Mutants of Streptococcus lactis C10, ML(3), and M18. Appl Environ Microbiol. 1979 Jun;37(6):1193–1195. doi: 10.1128/aem.37.6.1193-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Welch R. A. Transformation of Streptococcus sanguis with monomeric pVA736 plasmid deoxyribonucleic acid. J Bacteriol. 1981 May;146(2):826–830. doi: 10.1128/jb.146.2.826-830.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L. Functional properties of plasmids in lactic streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):259–274. doi: 10.1007/BF00399502. [DOI] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Slade H. D. Effect of filtrates from transformable and nontransformable streptococci on the transformation of streptococci. J Bacteriol. 1966 Jun;91(6):2216–2222. doi: 10.1128/jb.91.6.2216-2222.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Pathway of plasmid transformation in Pneumococcus: open circular and linear molecules are active. J Bacteriol. 1981 May;146(2):517–526. doi: 10.1128/jb.146.2.517-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Walsh P. M., McKay L. L. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J Bacteriol. 1981 Jun;146(3):937–944. doi: 10.1128/jb.146.3.937-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]






