Abstract
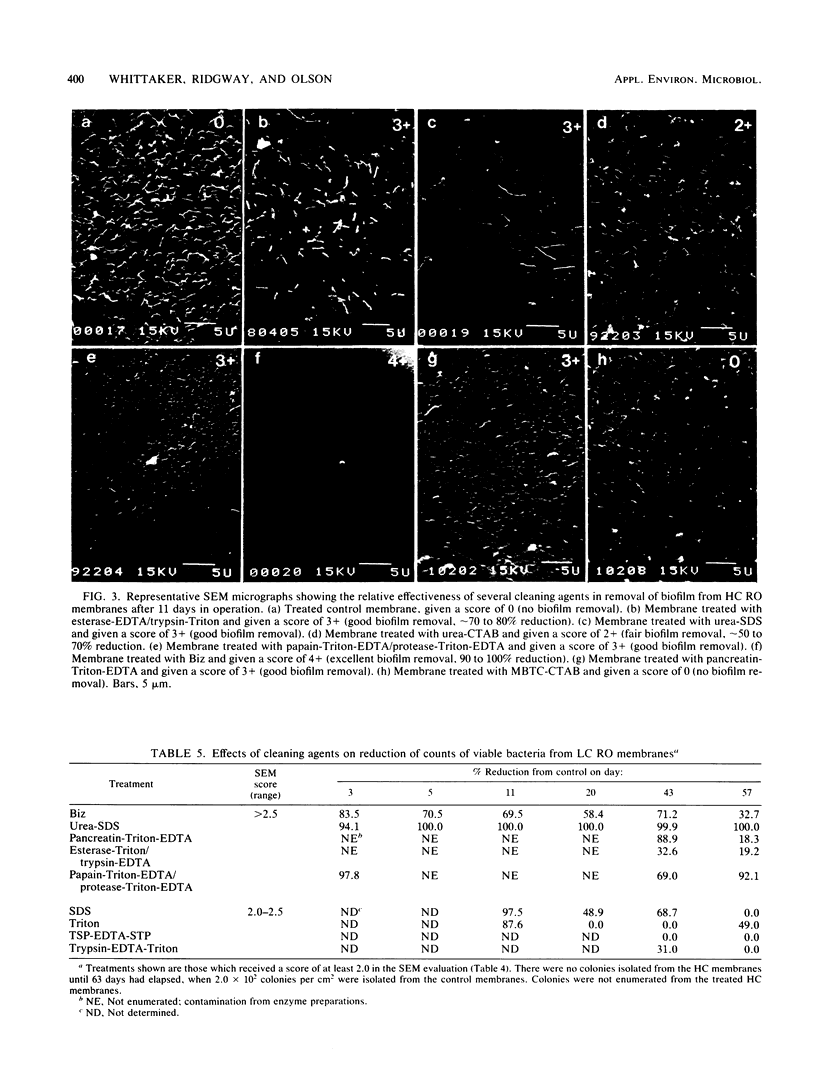
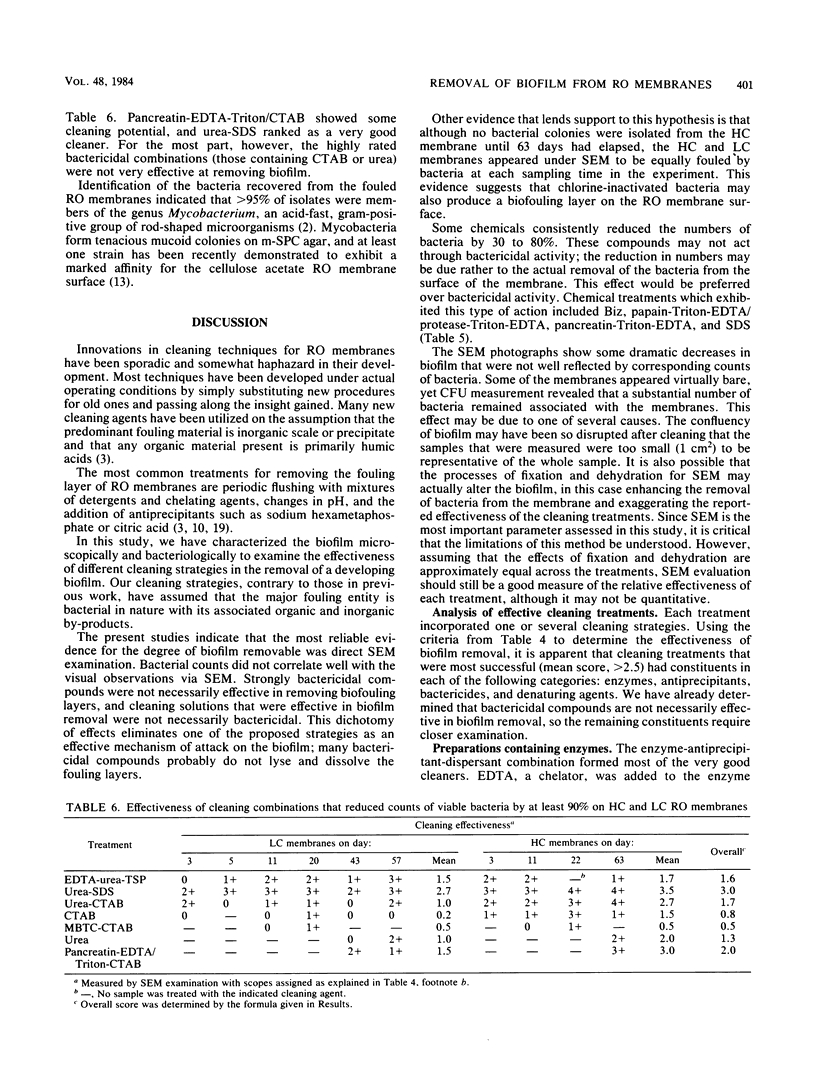
An evaluation was made of the efficiency of five classes of chemical cleaning agents for removing biofilm from spirally wound cellulose acetate reverse-osmosis membranes receiving influent with high or low levels of combined chlorine. Each cleaning regimen utilized one or more of the following types of chemical: (i) surfactants and detergents, (ii) chaotropic agents, (iii) bactericidal agents, (iv) enzymes, and (v) antiprecipitants. Cleaning efficiency was tested in the laboratory on membrane material removed from operations at various intervals (2 to 74 days). Cleaning effectiveness was evaluated against nontreated control membranes and was scored by scanning electron microscopy and enumeration of surviving bacteria after treatment of the membranes. The combinations of classes which were most effective in biofilm removal were the anionic and chaotropic agent combination and combinations involving enzyme-containing preparations. Membranes receiving influent with high levels of combined chlorine were easier to clean but more susceptible to structural damage from prolonged exposure to combined chlorine. No treatment or combination of treatments was completely effective or effective at all stages of biofilm development.
Full text
PDF
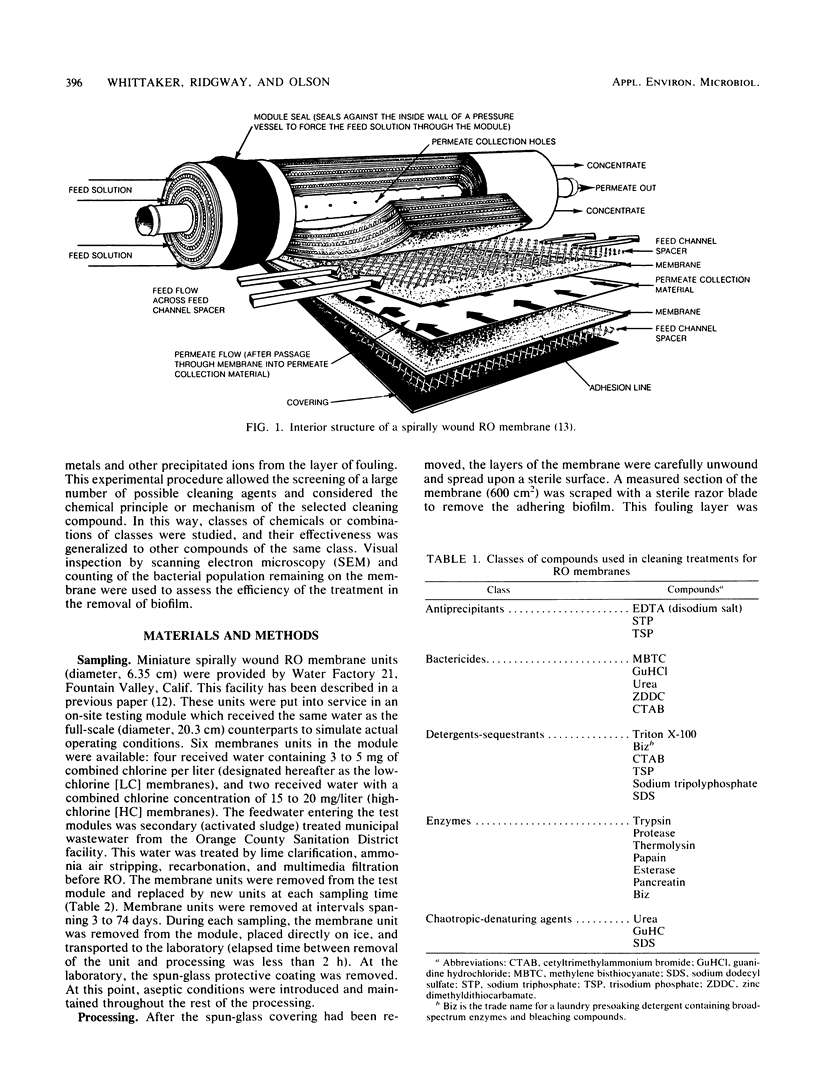
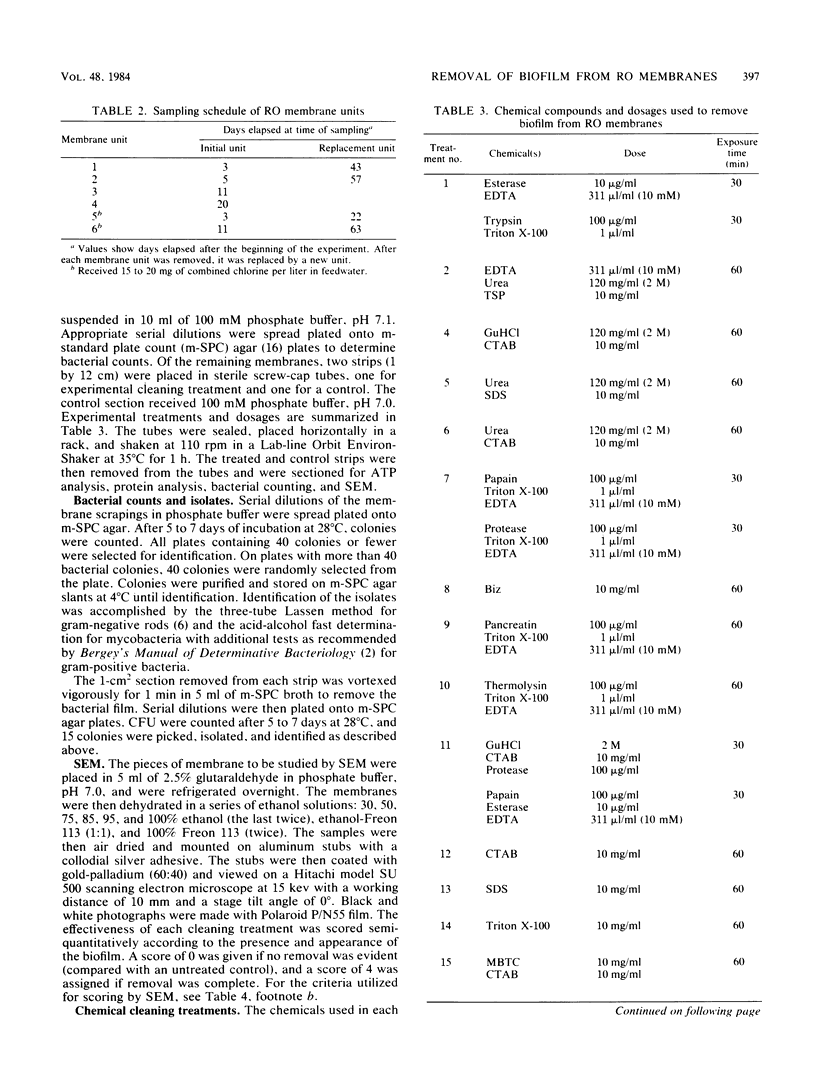
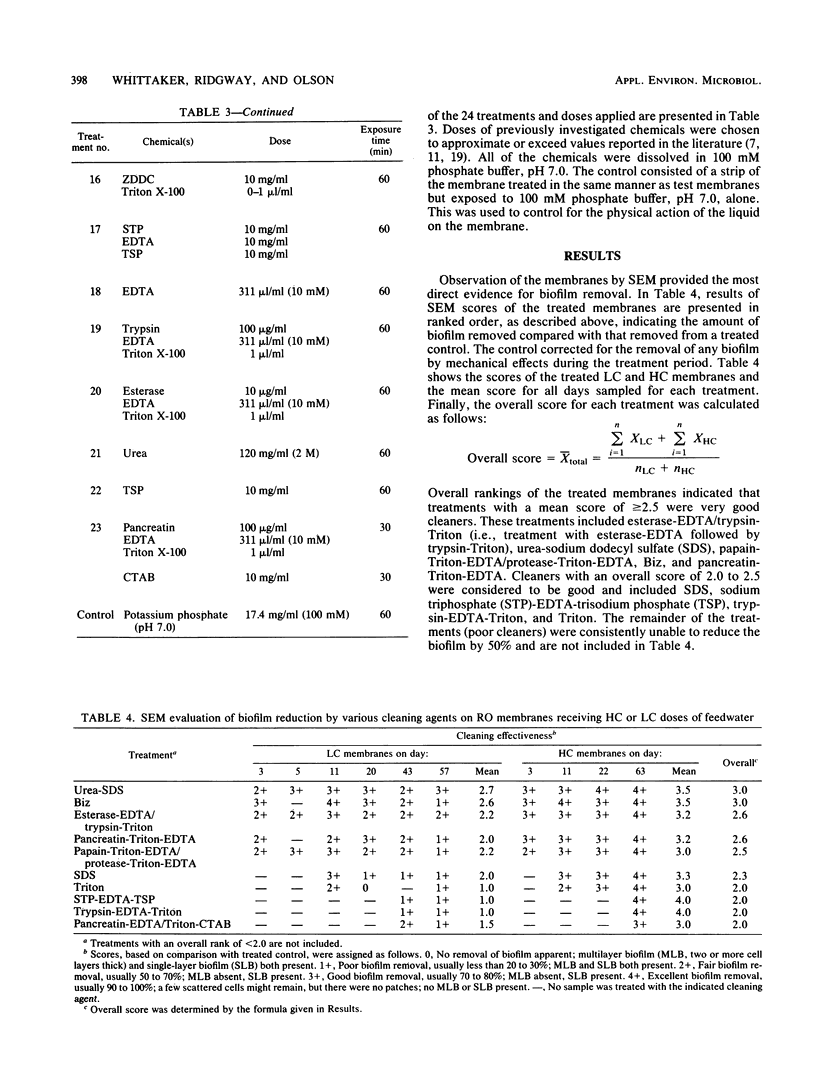
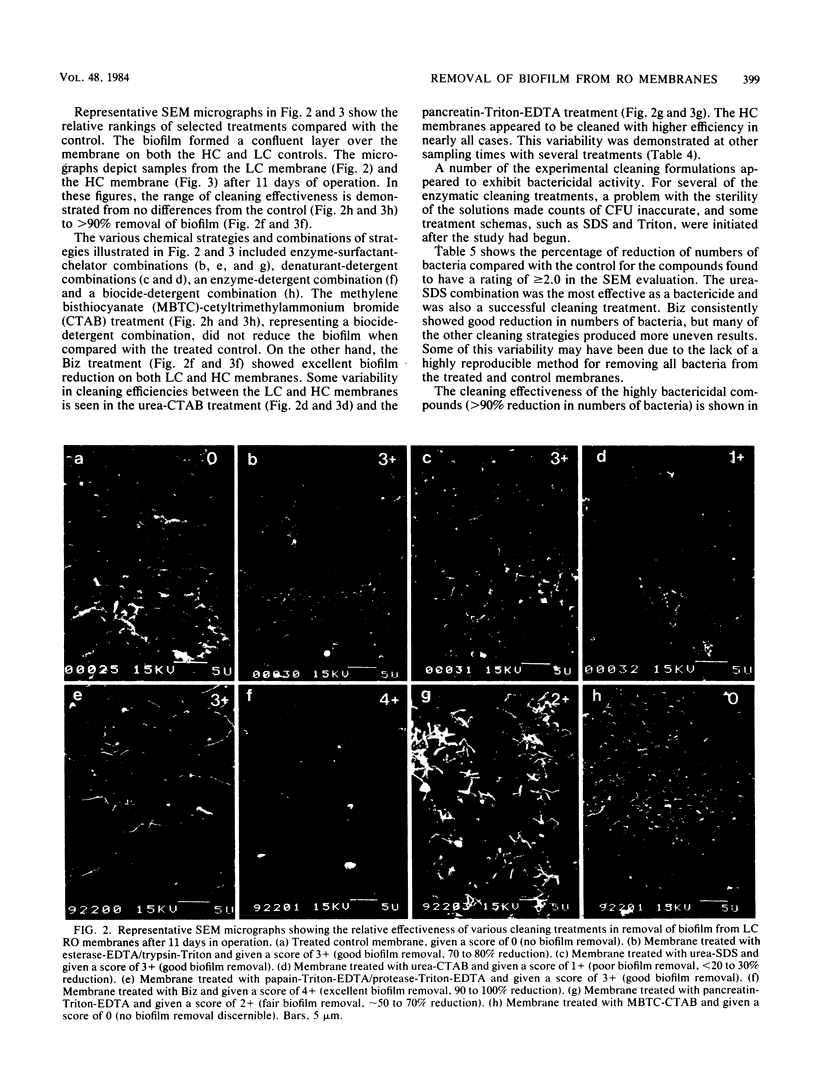


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GORDON J. A., JENCKS W. P. The relationship of structure to the effectiveness of denaturing agents for proteins. Biochemistry. 1963 Jan-Feb;2:47–57. doi: 10.1021/bi00901a011. [DOI] [PubMed] [Google Scholar]
- Lassen J. Rapid identification of gram-negative rods using a three-tube method combined with a dichotomic key. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):525–533. doi: 10.1111/j.1699-0463.1975.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Ridgway H. F., Kelly A., Justice C., Olson B. H. Microbial fouling of reverse-osmosis membranes used in advanced wastewater treatment technology: chemical, bacteriological, and ultrastructural analyses. Appl Environ Microbiol. 1983 Mar;45(3):1066–1084. doi: 10.1128/aem.45.3.1066-1084.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Rigby M. G., Argo D. G. Adhesion of a Mycobacterium sp. to cellulose diacetate membranes used in reverse osmosis. Appl Environ Microbiol. 1984 Jan;47(1):61–67. doi: 10.1128/aem.47.1.61-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




