Abstract
Widespread species- and genus-level extinctions of mammals in North America and Europe occurred during the last deglaciation [16,000–9,000 yr B.P. (by 14C)], a period of rapid and often abrupt climatic and vegetational change. These extinctions are variously ascribed to environmental change and overkill by human hunters. By contrast, plant extinctions since the Middle Pleistocene are undocumented, suggesting that plant species have been able to respond to environmental changes of the past several glacial/interglacial cycles by migration. We provide evidence from morphological studies of fossil cones and anatomical studies of fossil needles that a now-extinct species of spruce (Picea critchfieldii sp. nov.) was widespread in eastern North America during the Last Glacial Maximum. P. critchfieldii was dominant in vegetation of the Lower Mississippi Valley, and extended at least as far east as western Georgia. P. critchfieldii disappeared during the last deglaciation, and its extinction is not directly attributable to human activities. Similarly widespread plant species may be at risk of extinction in the face of future climate change.
Widespread Late Quaternary species- and genus-level extinctions of mammals in North America and Europe have been documented since the early 19th Century (1–4). These extinctions were concentrated during the last deglaciation [16,000 to 9,000 yr B.P. (by 14C)] (5, 6), a period of rapid and often abrupt climatic and vegetational change (7–9). In contrast to mammals, few Quaternary plant extinctions are documented (10–14). Most are concentrated at the Pliocene/Pleistocene boundary (ca. 1.6 million yr ago), although a few occurred in Europe during the Early to Middle Pleistocene. No Late Quaternary plant extinctions have been reported, leaving the impression that few or no extinctions occurred during the last glacial/interglacial cycle. Most knowledge of Late Quaternary vegetational and floristic change comes from fossil pollen preserved in lake and wetland sediments. Species extinctions may be masked by taxonomic smoothing in the pollen record, which rarely permits species-level differentiation. Plant macrofossils often support species-level identification, but few sites have been studied in detail, and few studies have included the detailed morphological and anatomical analyses required to determine whether fossil material is unequivocally assignable to extant or extinct species.
We provide evidence from morphological studies of fossil cones and anatomical studies of fossil needles for the Late Quaternary extinction of a species of Picea (spruce) that was widespread in the southeastern United States during, and immediately before, the Last Glacial Maximum. The organically preserved fossils are not assignable to any extant Picea species of North America; they occur at sites ranging from the Lower Mississippi Valley to the Atlantic Coastal Plain and Piedmont; and they are contemporaneous with fossils of extant eastern North American Picea species.
Most of the fossils are from streamcut exposures of Late Quaternary fluvial deposits in the Tunica Hills upland of Louisiana/Mississippi (31°N, 91°29′W; see Fig. 1). Silts and clays accumulated in low-energy backwater pools and abandoned channels (15, 16) contain abundant Picea megafossils, including ovulate cones, twigs, needles, and wood (17). Picea wood has been dated at 14 sites between 25,250 and 12,430 yr B.P. (16, 17). Megafossils of temperate taxa (Quercus spp., Juglans nigra, Acer sp., Carpinus caroliniana, Fagus grandifolia, Carya spp., Ulmus americana, and Juniperus virginiana) also occur in the deposits. Pollen assemblages dating 24,670 to 17,530 yr B.P. indicate that regional uplands were dominated by Picea, with small populations of Quercus and other hardwoods (17). The pollen assemblages lack modern analogs in eastern North America (18).
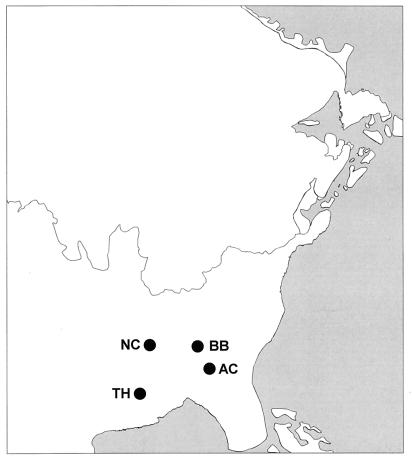
Figure 1.
Paleogeography of eastern North America at the time of the Last Glacial Maximum (18,000 yr B.P.). TH and BB represent Tunica Hills and Bob Black Pond, respectively, where Picea critchfieldii cones and/or foliage occur in sediments of the Last Glacial Maximum and Farmdalian Interstadial. AC and NC represent the Andersonville Claypit and Nonconnah Creek sites, respectively, where cones tentatively assigned to this species occur in Late Wisconsinan sediments.
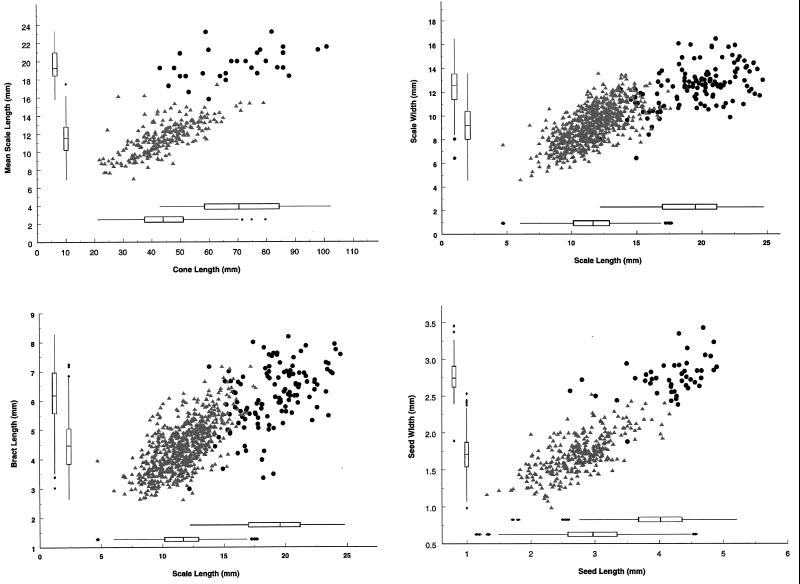
Ovulate cones from the deposits were originally identified as the boreal species Picea glauca (16, 19), although this identification was questioned by some observers (20, 21). The cones have been variously ascribed to an extinct species (21), or an extinct variety (22) or ecotype (23) of P. glauca. We examined 45 cones or cone fragments from eight Tunica Hills exposures (25,250 to 16,645 yr B.P.). Cone, scale, bract, and seed dimensions are larger than modern P. glauca, and are on different allometric trajectories (Figs. 2–4). Morphometric differences between cones of P. glauca and the fossil cones are at least as great as those between most pairs of extant North American Picea species (Table 1).
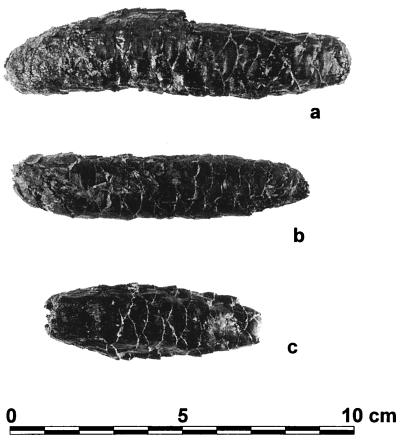
Figure 2.
Representative cones and cone fragments of P. critchfieldii from the Tunica Hills. (a) Holotype (TB-23/1093/15) from Tunica Bayou Site 23 (18,745 yr B.P.). (b) Paratype (PC-19/1191/1) from Pinckneyville Creek Site 19 (17,534 yr B.P.). (c) Apical fragment (PC-21/1092/4) from Pinckneyville Creek Site 21 (21,277 yr B.P.). Entire cone was >86 mm in length; basal portion was missing.
Figure 4.
Cone morphology of Tunica Hills fossils (from 20 cones or fragments from 6 sites; see ref. 17) and modern Picea glauca (from 240 cones from Alaska, South Dakota, Michigan, New York, Ontario, Saskatchewan, and Alberta). Box plots along margins portray median, quartiles, adjacent values, and outside values for the entire data sets. Upper box plots along x-axis and right-hand box plots along y-axis represent fossils, and lower box plots along x-axis and left-hand box plots along y-axis represent modern P. glauca. Scatter plots represent data for which paired values were available for the respective variables. Black dots represent fossils, and gray triangles represent modern P. glauca. Scales and attached bracts were obtained from the cone midpoint, 1/3 of the distance from the cone apex, and 1/3 of the distance from the cone base for each P. glauca cone. Number of scales and bracts measured from fossil cones varied from 3 to 25, depending on preservation. All were from the middle half of the cones. Seeds were removed from 12 fossil cones for measurement. Cone lengths for fossil specimens are minimum estimates in nearly all cases; most fossil cones were fragments lacking apical or basal portions.
Table 1.
Morphological and anatomical characteristics of ovulate cones and foliage needles of Picea critchfieldii and the 10 extant Picea species of North America
| Character | P. critchfieldii | P. glauca | P. mariana | P. rubens | P. engelmannii | P. pungens | P. sitchensis | P. breweriana | P. chihuahuana | P. martinezii | P. mexicana |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cone shape | Cylindrical | Cylindrical | Ovate | Ovate | Cylindrical | Cylindrical | Cylindrical | Oblanceolate | Cylindrical | Cylindrical | Cylindrical |
| Cone length, cm | >6.0–10.0 | 2.5–6.0 (−8.0) | 1.5–2.5 (−3.5) | 2.3–4.5 (−6.0) | 3.0–7.0 (−8.0) | 6.0–11.0 | 5.0–9.0 (−10.0) | 6.5–11.0 | 6.0–17.0 | 11.0–15.0 | 5.0–6.0 |
| Scale phyllotaxy | 3/5 | 3/5 | 3/5 | 3/5 | 5/8 | 5/8 | 5/8 | 3/5 | 5/8 | 5/8 | 3/5 |
| Scale shape | Fan | Fan | Fan | Fan | Elliptic/ diamond | Elliptic/ diamond | Elliptic/ diamond | Fan | Fan | Kite | Elliptic/ diamond |
| Scale length, mm | 18–21 (12–25) | 10–13 (5–17) | 8–11 | 8–14 (7–15) | 13–20 | 15–22 | 15–22 | 15–20 | 12–18 (10–22) | 25–27 | 12–14 |
| Scale width, mm | 11–14 (7–17) | 8–10 (4–14) | 7–9 | 7–11 (6–12) | 9–16 | 10–15 | 12–16 | 15–20 | 10–16 (7–18) | 21–23 | |
| Scale length:width | 1.4–1.7 (1.1–2.1) | 1.1–1.4 (0.8–1.9) | 0.9–1.25 | 1.1–1.4 (0.9–1.9) | 1.0–1.25 | 1.0–1.3 | 1.2–1.8 | ||||
| Bract length, mm | 5–7 (3–8) | 4–5 (2.5–7) | 1–2 | 3.5–6 (3–7) | 4.5–6.0 | 3.5–4.5 (3–5) | 4.5–5.5 | 5.5–6.0 | |||
| Bract length:scale length | 0.30–0.35 (0.15–0.50) | 0.35–0.45 (0.25–0.60) | 0.35–0.45 (0.30–0.55) | 0.3 | 0.25–0.35 | 0.2 | |||||
| Seed length, mm | 3.5–4.5 (2.5–5.2) | 2.5–3.5 (1.5–4.5) | |||||||||
| Needle apex | Acute | Acute | Blunt | Acute | Blunt | Acuminate | Acute | Blunt | Acuminate | Acuminate | Acuminate |
| Needle length, cm | 0.4–1.3 | 1.1–2.3 | 0.6–2.0 | 0.8–2.5 | 1.2–2.9 | 1.4–2.7 | 1.3–1.9 | 1.4–2.2 | 2.0–3.0 | 1.1–2.8 | 2.5–3.1 |
| Needle shape, transverse | Quadrangular | Quadrangular | Quadrangular | Quadrangular | Quadrangular | Quadrangular | Flattened | Flattened | Quadrangular | Quadrangular | Quadrangular |
| Lateral angle | Acute | Acute | Acute | Acute | Acute | Acute | Acute | Rounded | Rounded | Rounded | Acute |
| Resin ducts | Continuous | Intermittent | Continuous | Continuous | Intermittent | Intermittent | Intermittent | Continuous | Continuous | Continuous | Intermittent |
| Resin duct diam., μm | 70.6 ± 13.3 | 183.6 ± 43.6 | 137.0 ± 34.0 | 107.7 ± 18.5 | 244.9 ± 38.6 | 156.8 ± 36.8 | 176.8 ± 31.2 | 118.0 ± 17.9 | 52.1 ± 10.4 | 59.1 ± 16.6 | 250.5 ± 43.0 |
| Resin duct position | Angular | Lateral | Lateral | Lateral | Lateral | Lateral/ subangular | Lateral | Lateral | Angular | Angular | Lateral |
| Sclerified hypoderm | No | No | No | No | No | No | No | No | Yes | Yes | No |
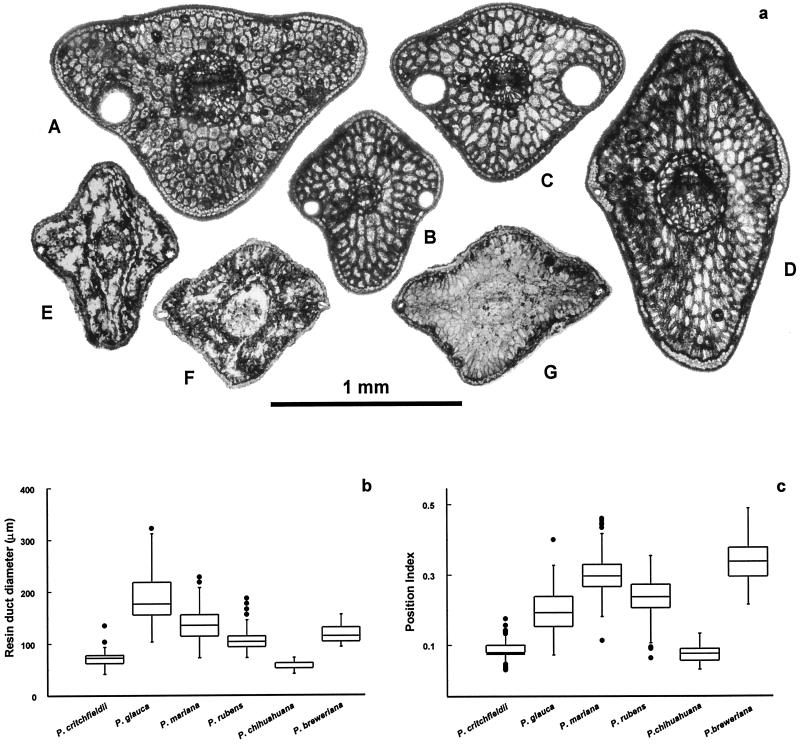
The fossil cones cannot be assigned to any of the other extant North American Picea species; they differ in shape, size, phyllotaxy, scale size and shape, and bract size (24–30) (Table 1). Neither are they assignable to the late Tertiary species Picea banksii (31), which differs in cone shape, phyllotaxy, and bract apex-shape. We also examined 50 foliage needles and needle fragments associated with the cones from six exposures (25,250 to 17,530 yr B.P.). None were assignable to any of the extant North American species, differing in apex shape, angularity, and hypoderm sclerification, as well as in size, continuity, and position of resin ducts (see ref. 32; Table 1; Fig. 5).
Figure 5.
(a) Needle cross-sections for selected North American Picea species, including A, P. glauca; B, P. rubens; C, P. mariana; D, P. chihuahuana; E–G, P. critchfieldii from Tunica Hills sediments. (b) Box plots showing resin-duct diameter of selected species. (c) Box plots showing resin-duct position index for selected species. The position index is the ratio of the distance of the resin duct from the lateral angle of the needle and the distance between adjacent needle angles (32).
Cones of P. glauca and Picea mariana occur at other sites that are contemporaneous with the Tunica Hills deposits (33, 34), and needles of both species are documented from contemporaneous sites to the north (C.W., S.T.J., and N. G. Miller, unpublished data). The occurrence in the Tunica Hills of morphologically unique cones in association with morphologically and anatomically unique needles, and the absence of other cone or needle morphotypes in the deposits indicates that they belong to a single, now-extinct species, Picea critchfieldii Jackson & Weng, sp. nov.
Picea critchfieldii Jackson & Weng, sp. nov.: Specific Diagnosis.
Ovulate cones cylindrical, 60(?)-100 mm long, 14–20 mm diameter. Scale phyllotaxy 3/5. Cone scales narrowly fan-shaped with rounded margins, 18–21 (12–25) mm long, 11–13.5 (7–16.5) mm wide; cone scale margin entire to moderately erose. Subtending bracts on cone scales spatulate, 5.5–7 (3.0–8.3) mm long, 1.5–1.8 (1.3–2.3) mm wide; bract apex rounded and minutely toothed. Seeds ovate, 3.5–4.5 (2.5–5.2) mm long, 2.6–2.8 (2.0–3.4) mm wide, each with a membranous wing 8–11 (4.5–13) mm long. Needles 7–9 (4–11) mm long, 0.6–1.0 mm diameter; quadrangular in cross-section; apex acute; two resin ducts spanning entire needle length; resin ducts 60–80 (40–100) μm in diameter, positioned in needle angles. Holotype: University of Wyoming Quaternary Plant Ecology Laboratory collections, TB-23/1093/15. Paratypes: PC-19/1191/1, PC-19/1191/8, TB-23/0393/8, TB-22/0293/1, PC-21/1092/1, PC-21/1092/2, PC-21/1092/4, LBS-1/0592/6, LBS-4/1191/5. Etymology: Named in honor of the late William B. Critchfield in recognition of his advocacy of understanding the role of Quaternary history in shaping genetic structure of conifer populations. He encouraged the senior author to investigate Quaternary spruce paleobotany and hence was partly responsible for initiating this study.
Discussion.
P. critchfieldii was not restricted to the Tunica Hills region (Fig. 1). Cones of similar dimension and morphology have been reported from sediments dating to 17,195 yr B.P. in western Tennessee (23), and 21,300 yr B.P. in southwestern Georgia (35) (W. B. Critchfield, personal communication, 1988; H. E. Cofer and J. Reinhardt, personal communication, 1989). We have identified needles of P. critchfieldii from sediments at Bob Black Pond in northwestern Georgia, co-occurring with Pinus rigida and Pinus banksiana during the Farmdalian interstadial (22,000–21,000 yr B.P.) and with Pinus banksiana, Pinus resinosa, Pinus strobus, and Picea glauca during the Last Glacial Maximum (21,000–16,500 yr B.P.). Occurrence at these sites (Fig. 1) indicates that P. critchfieldii had a range encompassing >240,000 km2 during the Late Wisconsinan.
The youngest fossils assignable to P. critchfieldii are cones from the Tunica Hills (16,645 yr B.P.) and needles from Bob Black Pond (16,620 yr B.P.). We have found no younger fossiliferous exposures in the Tunica Hills, and the record at Bob Black Pond is truncated at ca. 15,700 yr B.P. However, P. critchfieldii is the only Picea morphotype found in the Tunica Hills deposits, and other studies suggest that Picea persisted in the region to at least 12,430 yr B.P. (16). Picea populations were absent by 10,000 yr B.P. at sites further north in the Mississippi Valley (36). P. critchfieldii cones are absent from late-glacial and early Holocene macrofossil assemblages from deglaciated terrain in the mid-continent, where P. glauca and P. mariana cones are abundant (37). Evidently, P. critchfieldii never colonized these regions.
Paleoclimatic inferences from palynological data may be complicated by widespread occurrence of a now-extinct species during the Last Glacial Maximum. Extant eastern North American Picea species are boreal (P. glauca, P. mariana) or montane (Picea rubens), and all are associated with cool climates (mean July and January temperatures 8 to 21°C and −30 to 0°C, respectively) (38). An association of P. critchfieldii with temperate hardwoods in the Tunica Hills indicates warmer tolerances than the extant species. However, its occurrence with P. glauca and cool-temperate conifers at Bob Black Pond indicates some overlap in climate response with extant Picea species. Application of climate-response surface models, based on modern pollen assemblages, may yield erroneously low paleotemperature estimates for the Last Glacial Maximum in the southeastern region of the United States (18).
Pollen evidence indicates that Picea co-occurred with temperate tree taxa (Quercus, Carya, Fraxinus americana, Ulmus, and Ostrya/Carpinus), >500 km south of modern interglacial populations of Picea, during the Sangamonian and earlier interglacial periods (39–41). These assemblages are anomalous in terms of modern vegetation and pollen assemblages, and have been attributed to evolutionary changes in extant Picea species (42). Our results suggest that they may represent previous interglacial populations of P. critchfieldii, associated with many of the same taxa that were concurrent with it in the Tunica Hills during the Late Wisconsinan. Macrofossil studies from interglacial sediments can test this hypothesis.
In contrast to Late Quaternary vertebrate extinctions (4, 6, 43), the demise of P. critchfieldii cannot be ascribed to direct exploitation by humans. Attribution of its extinction to a particular event or process is difficult, especially in the absence of better paleobotanical data on its past geographic range and the time and places of its last occurrence. Many mechanisms might be responsible, including a pathogen, failure to disperse to and colonize newly available habitat as the environment changed, or permanent or temporary disappearance of its habitat space (44). Additional paleobotanical studies will help assess timing and causation of the extinction.
Paleoecological studies indicate that responses of plant species to the dramatic environmental changes of the Late Quaternary have consisted of continental-scale migrations (104 km) and changes in population density (37, 45–47). Many species have alternated between abundance and rarity during the past million years. Pinus ponderosa, which today forms extensive forests over much of the western North American interior, was restricted to scattered, isolated populations during the Last Glacial Maximum (47), as were most of the temperate hardwoods now dominant in eastern North America (18). Picea omorika, now restricted to small, scattered populations within a 750 km2 area in the Balkan region, appears to have been widespread in Europe during Early Quaternary interglacials (10).
Conclusions.
Our study indicates that extinction may be another outcome of environmental change. Species may be at significant risk of extinction as they respond to environmental change, especially when populations are reduced or fragmented by environmental restriction (44, 48). Although we provide here a clear documentation of a late Quaternary plant species extinction, other extinctions may be masked by the poor taxonomic resolution of the fossil pollen record and the paucity of detailed morphological studies of plant macrofossils. The late Quaternary disappearance of P. critchfieldii, after several thousand years of dominance in the Lower Mississippi Valley and widespread occurrence in the unglaciated southeastern United States, underscores the notion that “no species is safe” from extinction (49). This extinction is potentially sobering in view of the likelihood of future climatic changes, which could be of similar or greater rapidity, abruptness, and magnitude as those of the last glacial/interglacial transition (7–9, 50).
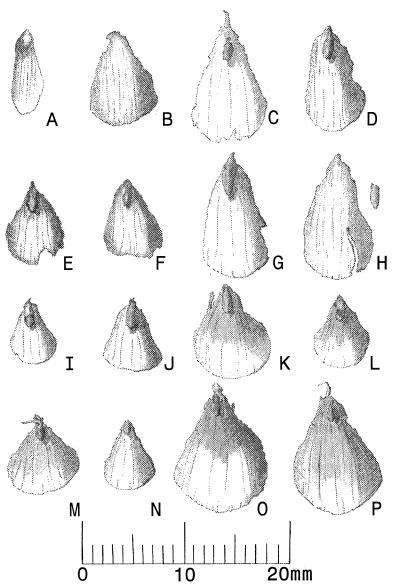
Figure 3.
(A) Seed and seedwing of P. critchfieldii (TB-23/1093/15). (B–H) Representative cone scales of P. critchfieldii from Tunica Hills sediments. B–D, G, and H are typical of scales from middle portions of cones. Some scales are abraded or are missing small portions, and bracts are missing from some specimens. (B) PC-19/1191/8; (C) TB-23/1093/15; (D) TB-23/0393/8; (E) PC-21/1092/1; (F) LBS-4/1191/5; (G and H) TB-22/0293/1. (I and J) Representative cone scales of modern P. glauca (I) Franklin Co., New York; (J) Cypress Hills, Saskatchewan). (K and L) Representative cone scales of modern Picea chihuahuana from Chihuahua, Mexico. (M and N) Representative cone scales of modern Picea breweriana from Siskiyou Co., California. (O and P) Representative cone scales of modern Picea martinezii from Nuevo Léon, Mexico.
Acknowledgments
We thank C. R. Givens for discussion and field assistance. Lab and field assistance was provided by K. Anderson, D. Boone, L. Lobaugh, J. Kearsley, M. Scherr, S. Stewart, B. Strauss, and C. Van Kirk. J. L. Betancourt, G. K. Brown, R. L. Hartman, B. Huntley, H. Iltis, J. T. Overpeck, T. Webb, III, and W. A. Watts provided discussion and encouragement. This study was supported by the National Science Foundation Climate Dynamics and Ecology Programs.
Abbreviation
- B.P.
time before the present, as calculated by 14C
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cuvier G. Discours sur les Révolutions de la Surface du Globe. Paris: Dufour and d’Ocagne; 1825. [Google Scholar]
- 2.Harlan R. Fauna Americana: Being a Description of the Mammiferous Animals Inhabiting North America. Philadelphia: Finley; 1825. [Google Scholar]
- 3.Lyell C. Am J Sci. 1844;46:320–323. [Google Scholar]
- 4.Grayson D K. In: Quaternary Extinctions: A Prehistoric Revolution. Martin P S, Klein R G, editors. Tucson: Univ. Arizona Press; 1984. pp. 5–39. [Google Scholar]
- 5.Mead J I, Meltzer D J. In: Quaternary Extinctions: A Prehistoric Revolution. Martin P S, Klein R G, editors. Tucson: Univ. Arizona Press; 1984. pp. 440–450. [Google Scholar]
- 6.Stuart A J. Biol Rev. 1991;66:453–562. doi: 10.1111/j.1469-185x.1991.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 7.Overpeck J T, Bartlein P J, Webb T., III Science. 1991;254:692–695. doi: 10.1126/science.254.5032.692. [DOI] [PubMed] [Google Scholar]
- 8.Taylor K C, Lamorey G W, Doyle G A, Alley R B, Grootes P M, Mayewski P A, White J W C, Barlow L K. Nature (London) 1993;361:432–436. [Google Scholar]
- 9.Broecker W S. Science. 1997;278:1582–1588. doi: 10.1126/science.278.5343.1582. [DOI] [PubMed] [Google Scholar]
- 10.Watts W A. In: Vegetation History. Huntley B, Webb T III, editors. Dordrecht, The Netherlands: Kluwer; 1988. pp. 155–192. [Google Scholar]
- 11.Tralau H. Bot Not. 1959;112:385–406. [Google Scholar]
- 12.Leopold E B. In: Pleistocene Extinctions: The Search for a Cause. Martin P S, Wright H E Jr, editors. New Haven, CT: Yale Univ. Press; 1967. pp. 203–246. [Google Scholar]
- 13.van der Hammen T, Wijmstra T A, Zagwijn W H. In: Late Cenozoic Glacial Ages. Turekian K K, editor. New Haven, CT: Yale Univ. Press; 1971. pp. 391–424. [Google Scholar]
- 14.Godwin H. The History of the British Flora. 2nd Ed. Cambridge, U.K.: Cambridge Univ. Press; 1975. [Google Scholar]
- 15.Delcourt P A, Delcourt H R. Quat Res. 1977;7:218–237. [Google Scholar]
- 16.Givens C R, Givens F M. Quat Res. 1987;27:283–296. [Google Scholar]
- 17.Jackson S T, Givens C R. Quat Res. 1994;41:316–325. [Google Scholar]
- 18.Jackson, S. T., Webb, R. S., Anderson, K. H., Overpeck, J. T., Webb, T. III, Williams, J. & Hansen, B. C. S. (2000) Quat. Sci. Rev., in press.
- 19.Brown C A. Louisiana Geol Surv Bull. 1938;12:59–96. [Google Scholar]
- 20.Whitehead D R. In: The Quaternary of the United States. Wright H E Jr, Frey D G, editors. Princeton, NJ: Princeton Univ. Press; 1965. pp. 417–432. [Google Scholar]
- 21.Critchfield W B. In: Proceedings of Eighth North American Forest Biology Workshop. Lanner R M, editor. Logan: Utah State Univ.; 1984. pp. 70–118. [Google Scholar]
- 22.Watts W A. In: Late-Quaternary Environments of the United States, Vol. 1, The Late Pleistocene. Porter S C, editor. Minneapolis: Univ. Minnesota Press; 1983. pp. 294–310. [Google Scholar]
- 23.Delcourt P A, Delcourt H R, Brister R C, Lackey L E. Quat Res. 1980;13:111–132. [Google Scholar]
- 24.Taylor R J Flora of North America Editorial Committee, editors. Flora of North America North of Mexico, Vol. 2: Pteridophytes and Gymnosperms. New York: Oxford Univ. Press; 1993. pp. 369–373. [Google Scholar]
- 25.Schmidt-Vogt H. Die Fichte. Band I. Taxonomie, Verbreitung, Morphologie, Ökologie, Waldgesellschaften. Hamburg, Germany: Paul Parey; 1977. [Google Scholar]
- 26.Dugle J R, Bols N. Variation in Picea glauca and P. mariana in Manitoba and Adjacent Areas. Whiteshell Nuclear Research Establishment, Pinawa, Ontario: Atomic Energy of Canada; 1971. [Google Scholar]
- 27.Parker W H, McLachlan D G. Can J Bot. 1978;56:2512–2520. [Google Scholar]
- 28.Martínez M. Las Pináceas Mexicanas. 3rd Ed. México: Univ. Nac. Aut. México; 1963. [Google Scholar]
- 29.Daubenmire R. Can J Bot. 1974;52:1545–1560. [Google Scholar]
- 30.Patterson T E. SIDA. 1988;13:131–135. [Google Scholar]
- 31.Hills L V, Ogilvie R T. Can J Bot. 1970;48:457–464. [Google Scholar]
- 32.Weng C. Ph.D. thesis. Laramie: Univ. of Wyoming; 1998. [Google Scholar]
- 33.Jaumann P J. M.S. thesis. Lawrence: Univ. of Kansas; 1989. [Google Scholar]
- 34.Baker R G, Rhodes R S, II, Schwert D P, Ashworth A C, Frest T J, Hallberg G R, Janssens J A. J Quat Sci. 1986;1:91–107. [Google Scholar]
- 35.Cofer H E, Manker J P. U.S. Geol. Surv. Open-File Rep. 1983. 83–580. [Google Scholar]
- 36.Delcourt P A, Delcourt H R. Eng Geol. 1996;45:219–242. [Google Scholar]
- 37.Jackson S T, Overpeck J T, Webb T, III, Keattch S E, Anderson K H. Quat Sci Rev. 1997;16:1–70. [Google Scholar]
- 38.Thompson R S, Anderson K H, Bartlein P J. Atlas of Relations Between Climatic Parameters and Distributions of Important Trees and Shrubs in North America. Washington, DC: U.S. Geol. Surv.; 1999. , Prof. Paper 1650. [Google Scholar]
- 39.Kapp R O, Gooding A M. J Geol. 1964;72:307–326. [Google Scholar]
- 40.King J E, Saunders J J. Quat Res. 1986;25:89–99. [Google Scholar]
- 41.Nickmann R J, Demarest J M, II. Quat Res. 1982;17:93–104. [Google Scholar]
- 42.Kapp R O. In: Geobotany. Romans R C, editor. New York: Plenum; 1977. pp. 1–26. [Google Scholar]
- 43.Martin P S, Klein R G, editors. Quaternary Extinctions: A Prehistoric Revolution. Tucson: Univ. Arizona Press; 1984. [Google Scholar]
- 44.Huntley B. In: Conservation in a Changing World. Mace G M, Balmford A, Ginsberg J R, editors. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 69–85. [Google Scholar]
- 45.Davis M B. In: Late-Quaternary Environments of the United States, Vol. 2, The Holocene. Wright H E Jr, editor. Minneapolis: Univ. Minnesota Press; 1983. pp. 166–181. [Google Scholar]
- 46.Huntley B, Webb T., III J Biogeogr. 1989;16:5–19. [Google Scholar]
- 47.Betancourt J L, Van Devender T R, Martin P S. In: Packrat Middens: The Last 40,000 Years of Biotic Change. Betancourt J L, Van Devender T R, Martin P S, editors. Tucson: Univ. Arizona Press; 1990. pp. 435–447. [DOI] [PubMed] [Google Scholar]
- 48.Delcourt P A, Delcourt H R Flora of North America Editorial Committee, editors. Flora of North America North of Mexico, Vol. 1: Introduction. New York: Oxford Univ. Press; 1993. pp. 71–94. [Google Scholar]
- 49.Rosenzweig M L. Species Diversity in Space and Time. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 50.Houghton J T, Meira Filho L G, Callander B A, Harris N, Kattenberg A, Maskell K, editors. Climate Change 1995: The Science of Climate Change. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]