Abstract
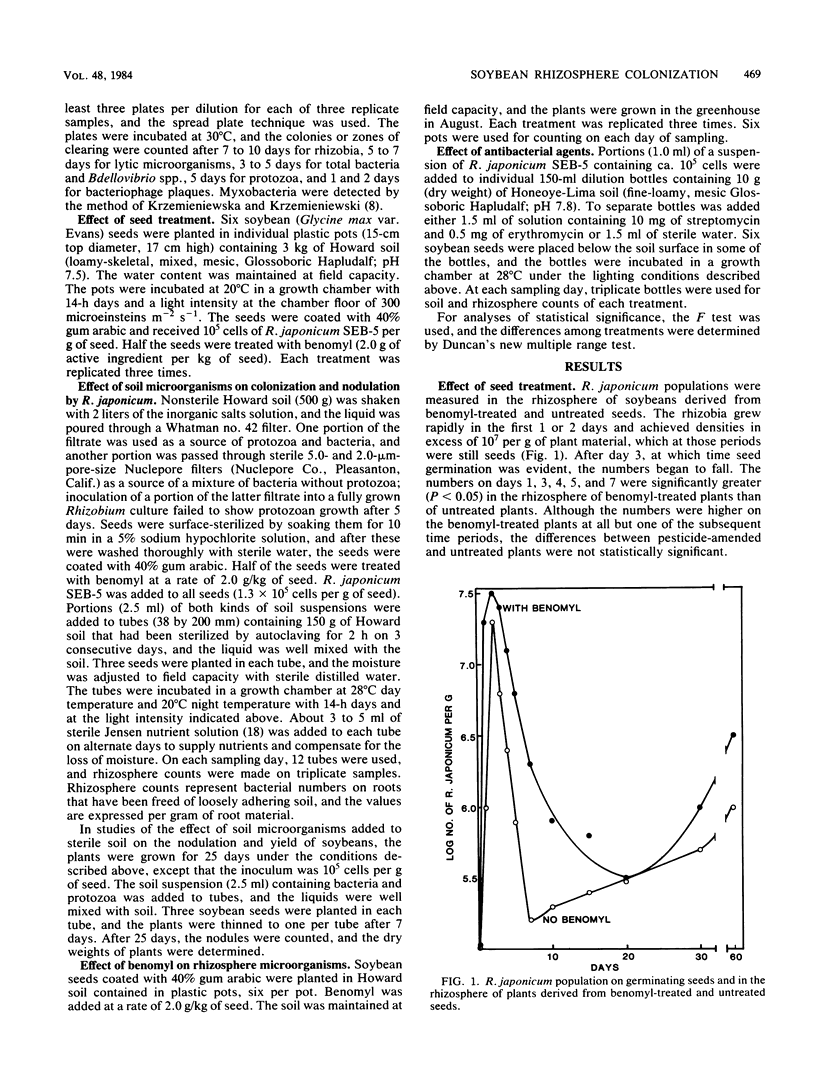
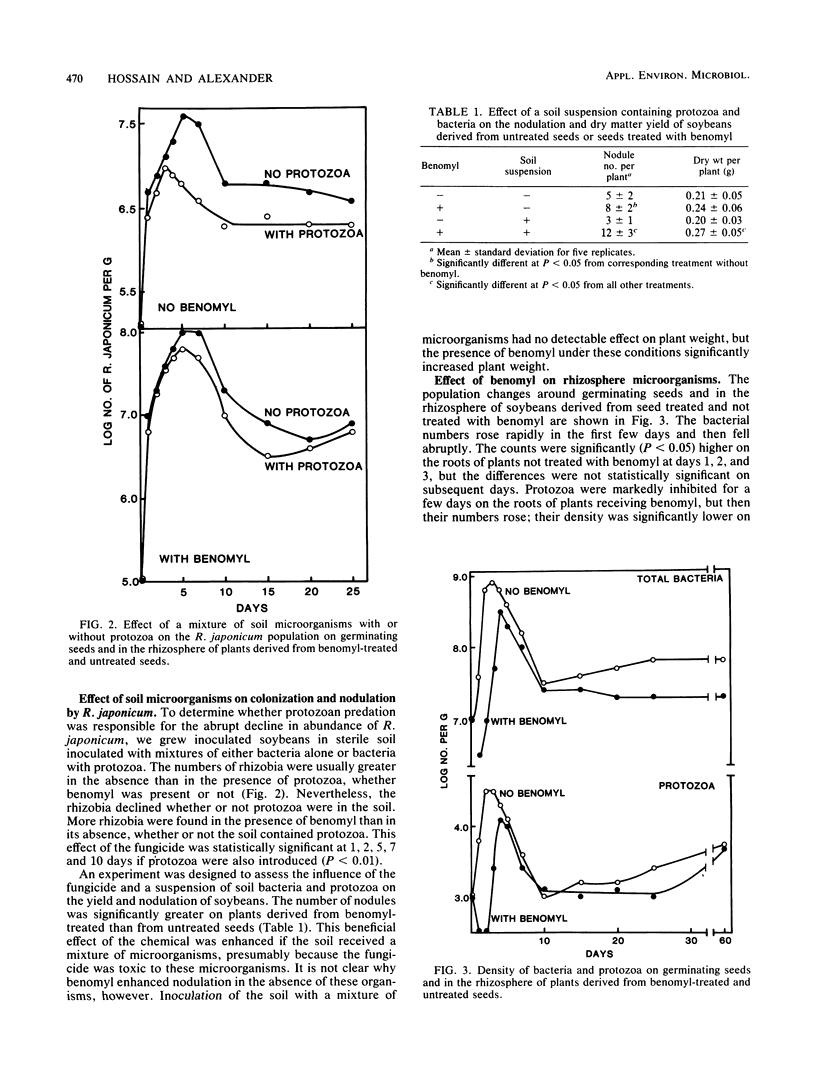
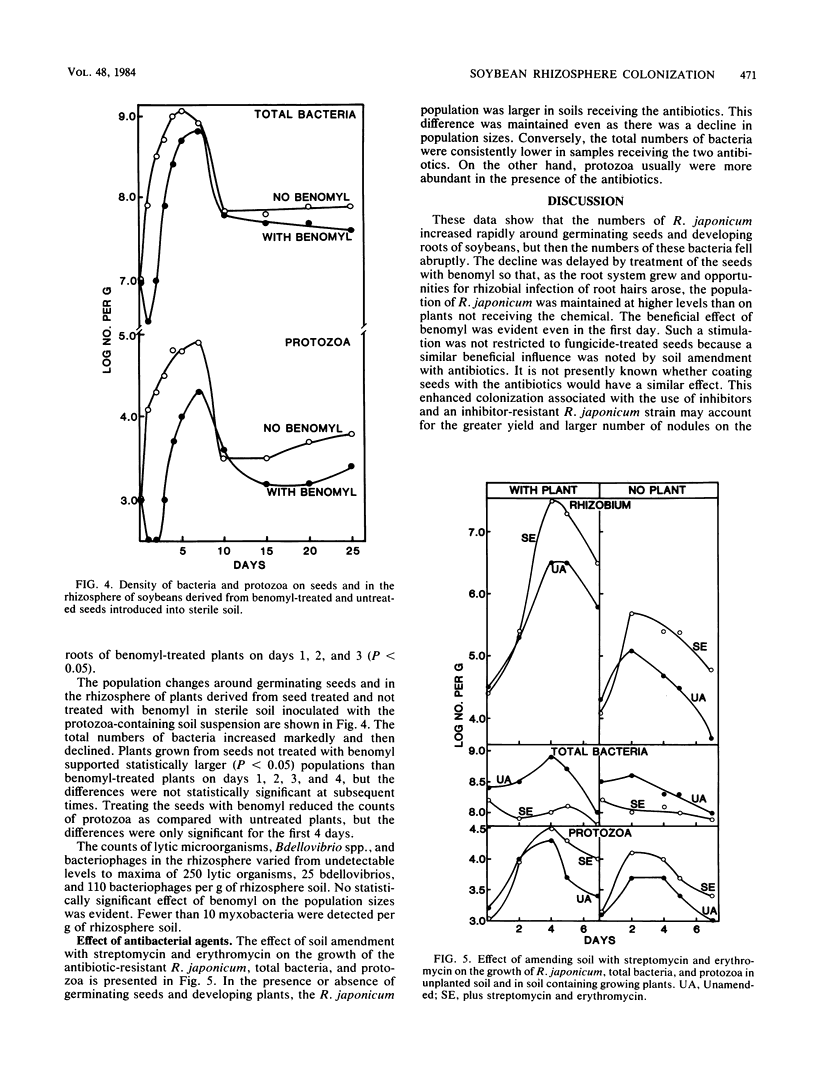
A study was conducted to seek means to increase the colonization of the rhizosphere of soybeans (Glycine max L. Merrill) by Rhizobium japonicum. For this purpose, a strain of R. japonicum that was resistant to benomyl, streptomycin, and erythromycin was used. The numbers of R. japonicum rose quickly in the first 2 days after soybean seeds were planted in soil and then rapidly fell. The decline was slower if the seeds were coated with benomyl. This fungicide reduced the numbers of bacteria and protozoa in the rhizosphere, but the effect became less or disappeared as the plants grew. In sterile soil inoculated with R. japonicum and a mixture of microorganisms, the numbers of R. japonicum were usually lower if protozoa were present than if they were absent. Nodulation and plant yield were increased by the addition of benomyl to soybean seeds sown in sterile soil inoculated with R. japonicum and a mixture of microorganisms. The addition of streptomycin and erythromycin to soil stimulated the growth of R. japonicum but inhibited other bacteria in the presence or absence of soybeans. The data indicate that colonization can be increased by the use of antimicrobial agents and R. japonicum strains resistant to those inhibitors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOK F. D., QUADLING C. A modified technique for isolation of bacteriophage from contaminated materials. Can J Microbiol. 1959 Jun;5(3):311–312. doi: 10.1139/m59-037. [DOI] [PubMed] [Google Scholar]
- Danso S. K., Habte M., Alexander M. Estimating the density of individual bacterial populations introduced into natural ecosytems. Can J Microbiol. 1973 Nov;19(11):1450–1451. doi: 10.1139/m73-234. [DOI] [PubMed] [Google Scholar]
- Habte M., Alexander M. Further evidence for the regulation of bacterial populations in soil by protozoa. Arch Microbiol. 1977 Jun 20;113(3):181–183. doi: 10.1007/BF00492022. [DOI] [PubMed] [Google Scholar]
- Lennox L. B., Alexander M. Fungicide Enhancement of Nitrogen Fixation and Colonization of Phaseolus vulgaris by Rhizobium phaseoli. Appl Environ Microbiol. 1981 Feb;41(2):404–411. doi: 10.1128/aem.41.2.404-411.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Castro F. A., Alexander M. Method for establishing a bacterial inoculum on corn roots. Appl Environ Microbiol. 1983 Jan;45(1):248–254. doi: 10.1128/aem.45.1.248-254.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez C., Alexander M. Evidence Suggesting Protozoan Predation on Rhizobium Associated with Germinating Seeds and in the Rhizosphere of Beans (Phaseolus vulgaris L.). Appl Environ Microbiol. 1980 Sep;40(3):492–499. doi: 10.1128/aem.40.3.492-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLP H., STARR M. P. BDELLOVIBRIO BACTERIOVORUS GEN. ET SP. N., A PREDATORY, ECTOPARASITIC, AND BACTERIOLYTIC MICROORGANISM. Antonie Van Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]


