Abstract
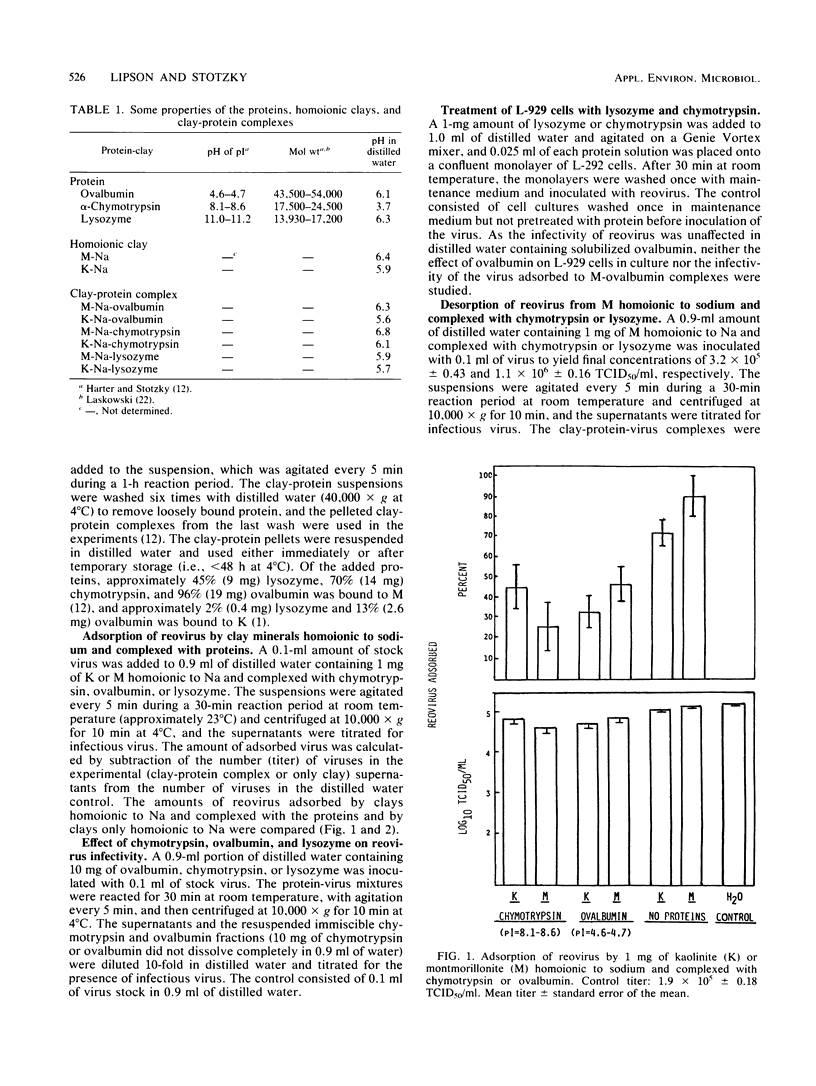
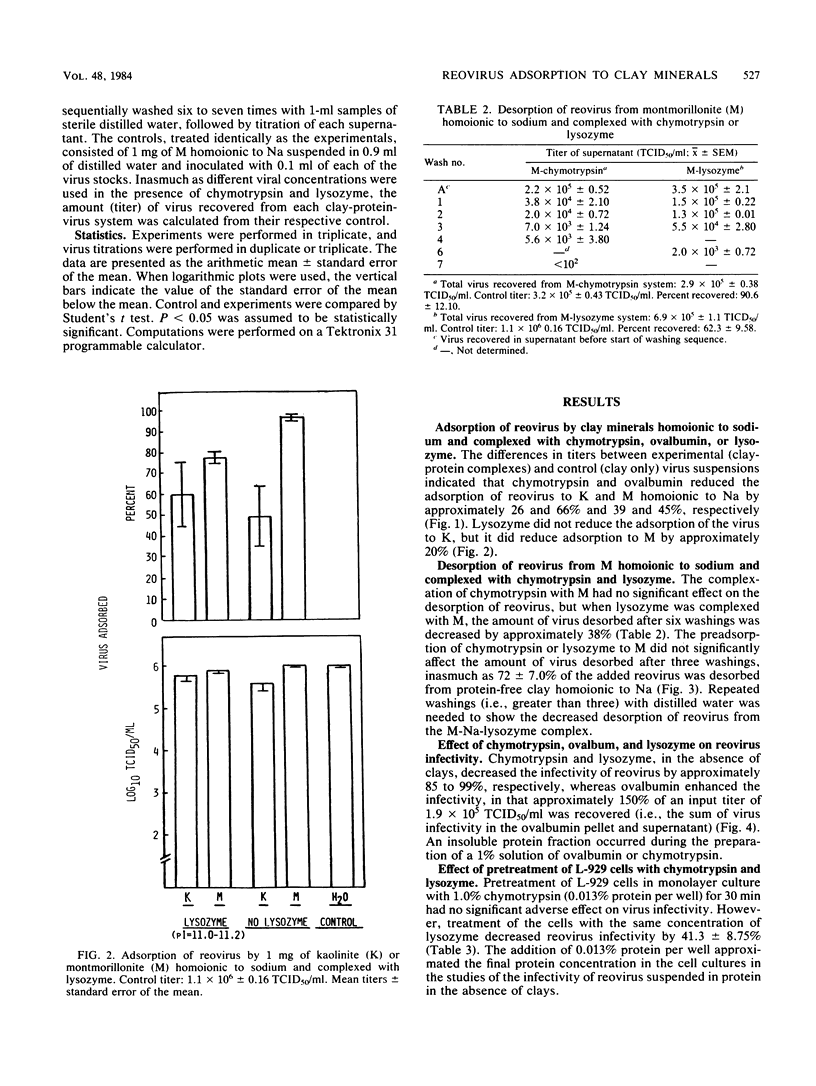
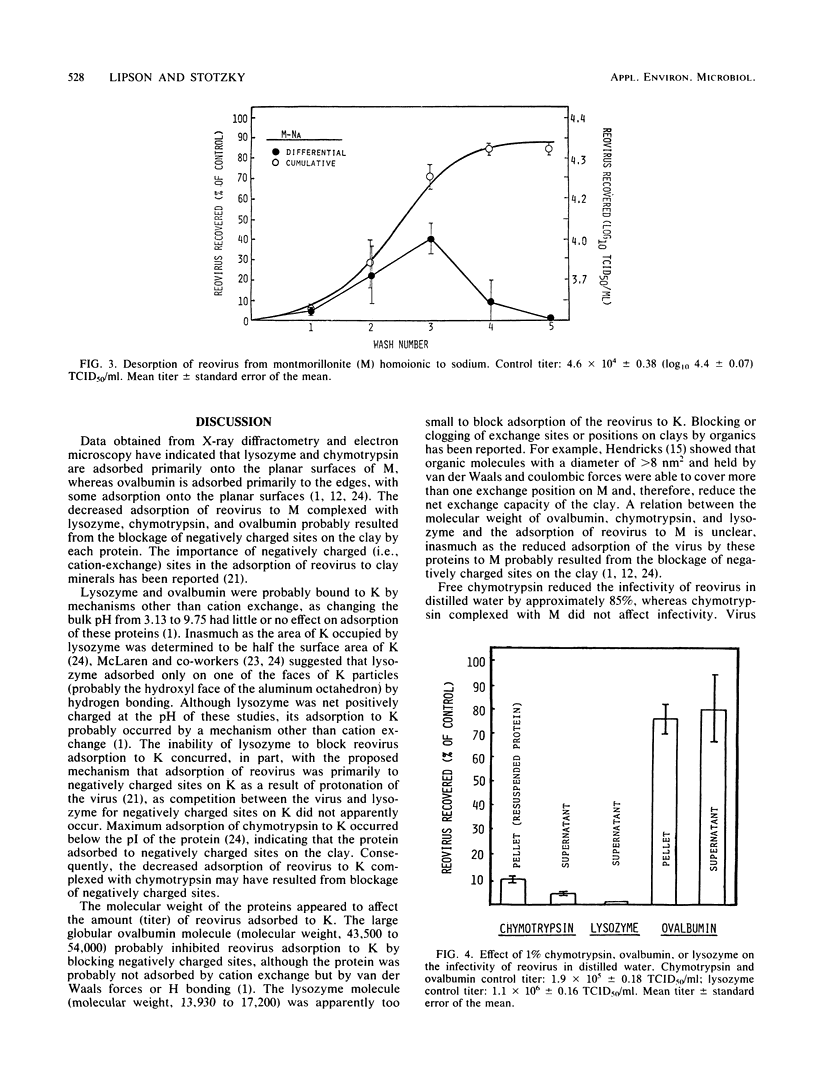
Organic matter in sewage, soil, and aquatic systems may enhance or inhibit the infectivity of viruses associated with particulates (e.g., clay minerals, sediments). The purpose of this investigation was to identify the mechanisms whereby organic matter, in the form of defined proteins, affects the adsorption of reovirus to the clay minerals kaolinite and montmorillonite and its subsequent infectivity. Chymotrypsin and ovalbumin reduced the adsorption of reovirus to kaolinite and montmorillonite homoionic to sodium. Lysozyme did not reduce the adsorption of the virus to kaolinite, but it did reduce adsorption to montmorillonite. The proteins apparently competed with the reovirus for sites on the clay. As lysozyme does not adsorb to kaolinite by cation exchange, it did not inhibit the adsorption of reovirus to this clay. The amount of reovirus desorbed from lysozyme-coated montmorillonite was approximately 38% less (compared with the input population) than that from uncoated or chymotrypsin-coated montmorillonite after six washings with sterile distilled water. Chymotrypsin and lysozyme markedly decreased reovirus infectivity in distilled water, whereas infectivity of the virus was enhanced after recovery from an ovalbumin-distilled water-reovirus suspension (i.e., from the immiscible pelleted fraction plus supernatant). The results of these studies indicate that the persistence of reovirus in terrestrial and aquatic environments may vary with the type of organic matter and clay mineral with which the virus comes in contact.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane C. G., Griffin J. H. The biochemistry and pathophysiology of the contact system of plasma. Adv Immunol. 1982;33:241–306. doi: 10.1016/s0065-2776(08)60837-8. [DOI] [PubMed] [Google Scholar]
- Drewry W. A., Eliassen R. Virus movement in groundwater. J Water Pollut Control Fed. 1968 Aug;(Suppl):257–271. [PubMed] [Google Scholar]
- Duboise S. M., Moore B. E., Sorber C. A., Sagik B. P. Viruses in soil systems. CRC Crit Rev Microbiol. 1979 Nov;7(3):245–301. doi: 10.3109/10408417909082016. [DOI] [PubMed] [Google Scholar]
- Filip Z. Clay minerals as a factor influencing the biochemical activity of soil microorganisms. Folia Microbiol (Praha) 1973;18(1):56–74. doi: 10.1007/BF02884250. [DOI] [PubMed] [Google Scholar]
- Gerba C. P., Schaiberger G. E. Effect of particulates on virus survival in seawater. J Water Pollut Control Fed. 1975 Jan;47(1):93–103. [PubMed] [Google Scholar]
- Hattori T., Hattori R. The physical environment in soil microbiology: an attempt to extend principles of microbiology to soil microoganisms. CRC Crit Rev Microbiol. 1976 May;4(4):423–461. doi: 10.3109/10408417609102305. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., McDevitt P. J. The binding of bovine factor XII to kaolin. Blood. 1983 Apr;61(4):652–659. [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl Environ Microbiol. 1979 Jul;38(1):93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry E. F., Vaughn J. M., Vicale T. J., Mann R. Accumulation of sediment-associated viruses in shellfish. Appl Environ Microbiol. 1983 Jan;45(1):238–247. doi: 10.1128/aem.45.1.238-247.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson S. M., Stotzky G. Adsorption of reovirus to clay minerals: effects of cation-exchange capacity, cation saturation, and surface area. Appl Environ Microbiol. 1983 Sep;46(3):673–682. doi: 10.1128/aem.46.3.673-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAMOTO Y. Further evidence for the longevity of soil-borne plant viruses adsorbed on soil particles. Virology. 1959 Oct;9:290–291. doi: 10.1016/0042-6822(59)90121-7. [DOI] [PubMed] [Google Scholar]
- Schaub S. A., Bausum H. T., Taylor G. W. Fate of virus in wastewater applied to slow-infiltration land treatment systems. Appl Environ Microbiol. 1982 Aug;44(2):383–394. doi: 10.1128/aem.44.2.383-394.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P., Melnick J. L. Role of sediment in the persistence of enteroviruses in the estuarine environment. Appl Environ Microbiol. 1978 Apr;35(4):685–689. doi: 10.1128/aem.35.4.685-689.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Dean C. H., Knuckles M. E., Wagner R. A. Interactions and survival of enteric viruses in soil materials. Appl Environ Microbiol. 1980 Jul;40(1):92–101. doi: 10.1128/aem.40.1.92-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. J., Rosenbaum M. J. Methods for preparing tissue culture in disposable microplates and their use in virology. Am J Epidemiol. 1967 May;85(3):424–437. [PubMed] [Google Scholar]



