Abstract
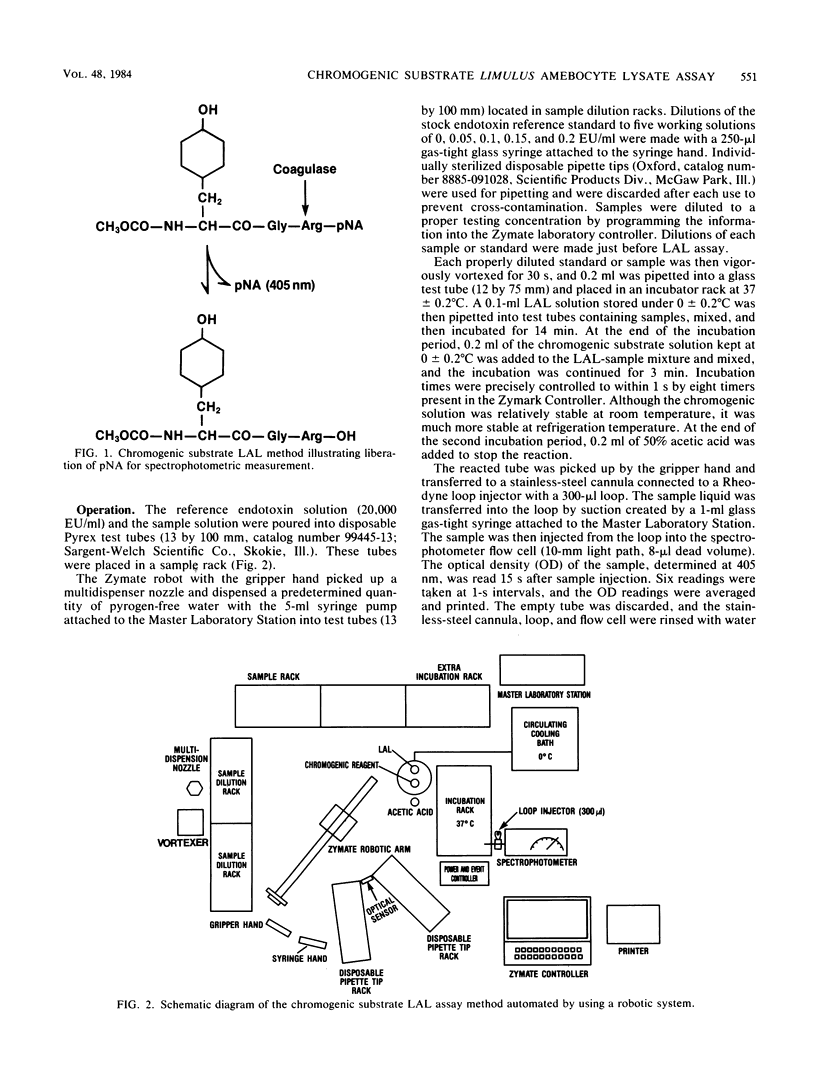
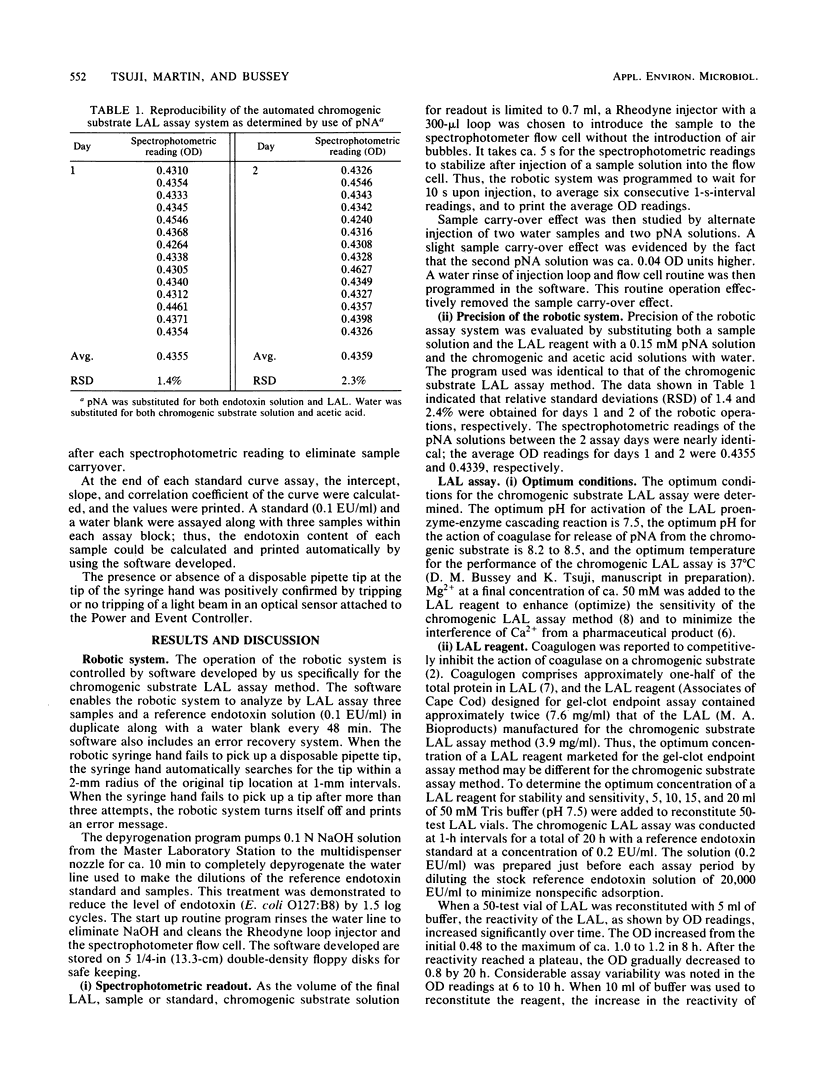
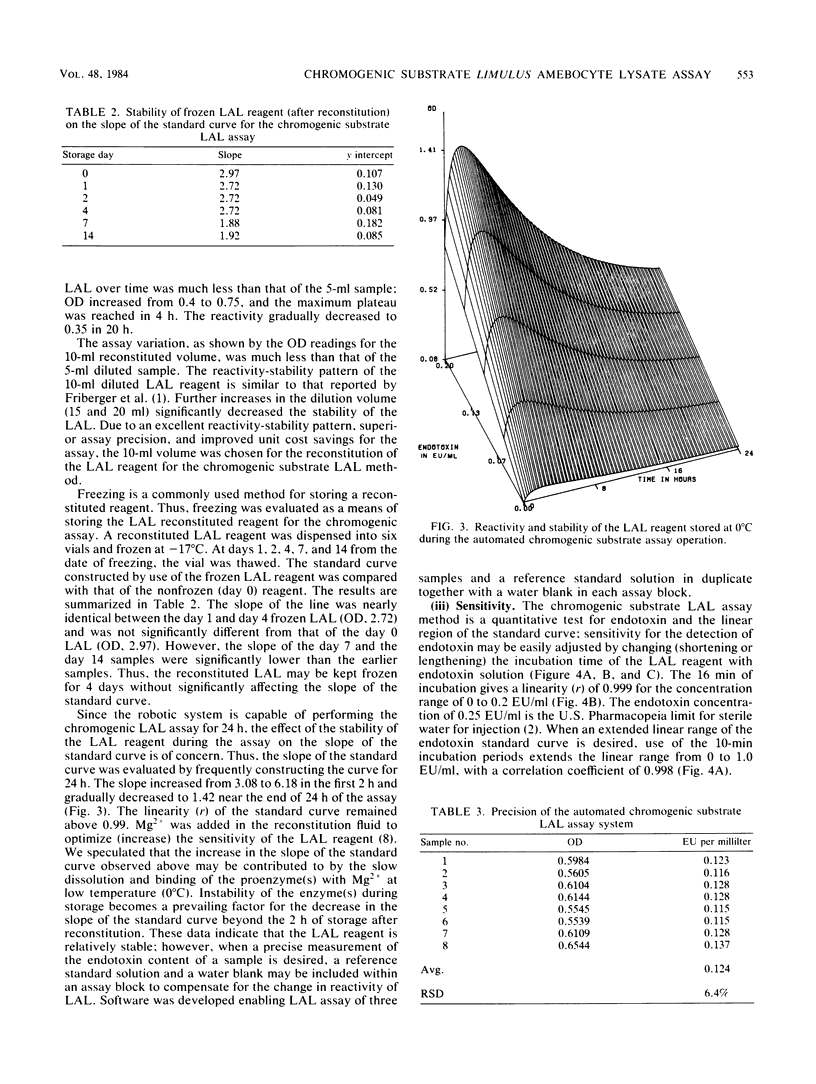
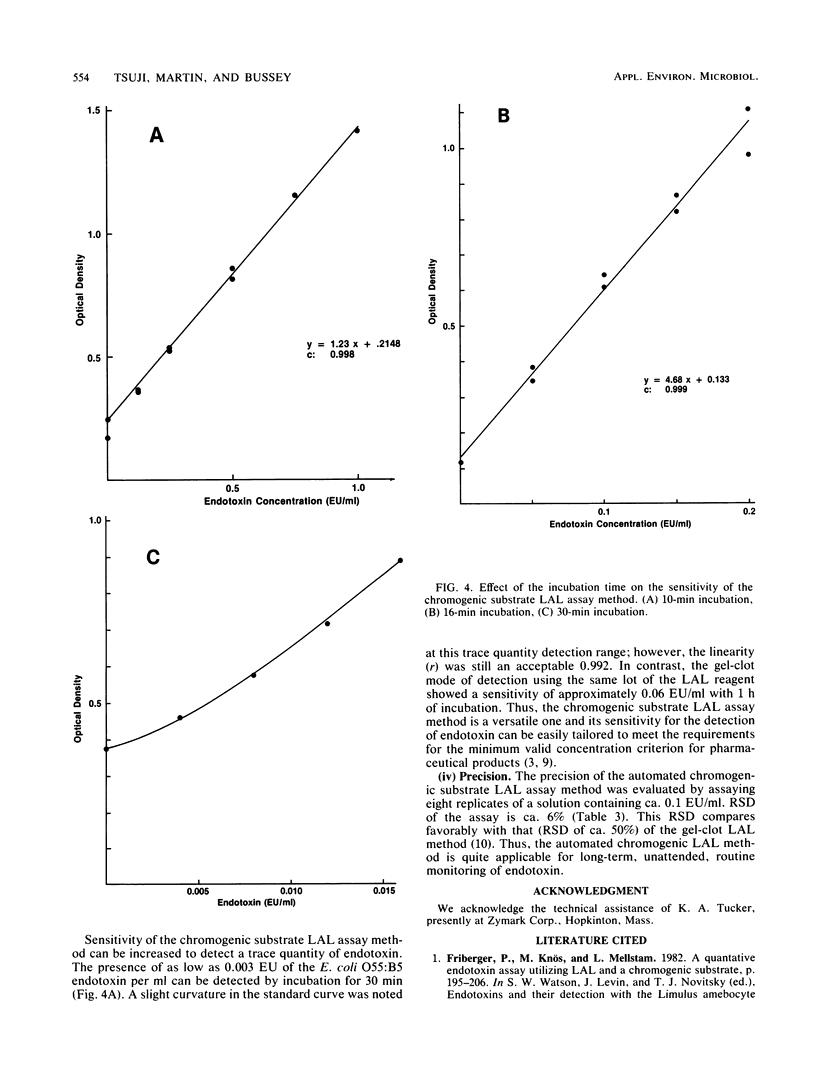
The chromogenic substrate Limulus amebocyte lysate (LAL) assay method for the detection of endotoxin was automated by a Zymate robotic system. The software developed enables the robot to automatically dilute a stock reference endotoxin standard (20,000 endotoxin units per ml) for the construction of a five-point standard curve, make sample dilutions to the proper testing concentration, and perform chromogenic substrate LAL assays in duplicate. The linearity of the standard curve and the endotoxin concentration in each sample are calculated and results are printed automatically. In 48 min the automated system assays three samples and a reference standard in duplicate along with a water blank. Sensitivity of the assay is a function of incubation time. The assay is linear (r greater than 0.99) in the region of 0 to 1.0 endotoxin units per ml or 0 to 0.2 endotoxin units per ml with incubation times of 10 or 16 min, respectively. The method can be made very sensitive, detecting as low as 0.003 endotoxin units per ml with 30 min of incubation. The precision of the assay method, determined by assaying an endotoxin reference solution eight times, is ca. 6%. The LAL reagent designed for gel-clot assay was modified for the chromogenic substrate assay. We describe the optimum conditions for the performance of the chromogenic substrate LAL assay and stability of the LAL reagent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friberger P., Knös M., Mellstam L. A quantitative endotoxin assay utilizing LAL and a chromogenic substrate. Prog Clin Biol Res. 1982;93:195–206. [PubMed] [Google Scholar]
- Harada-Suzuki T., Morita T., Iwanaga S., Nakamura S., Niwa M. Further studies on the chromogenic substrate assay method for bacterial endotoxins using horseshoe crab (Tachypleus tridentatus) hemocyte lysate. J Biochem. 1982 Sep;92(3):793–800. doi: 10.1093/oxfordjournals.jbchem.a133991. [DOI] [PubMed] [Google Scholar]
- Munson T. E. FDA guideline for validation of the LAL test as an end-product endotoxin test for human and biological drugs. Prog Clin Biol Res. 1982;93:25–32. [PubMed] [Google Scholar]
- Nakamura S., Takagi T., Iwanaga S., Niwa M., Takahashi K. Amino acid sequence studies on the fragments produced from horseshoe crab coagulogen during gel formation: homologies with primate fibrinopeptide B. Biochem Biophys Res Commun. 1976 Oct 4;72(3):902–908. doi: 10.1016/s0006-291x(76)80217-3. [DOI] [PubMed] [Google Scholar]
- Ohki M., Nakamura T., Morita T., Iwanaga S. A new endotoxin sensitive factor associated with hemolymph coagulation system of horseshoe crab (Limulidae). FEBS Lett. 1980 Nov 3;120(2):217–220. doi: 10.1016/0014-5793(80)80301-2. [DOI] [PubMed] [Google Scholar]
- Steindler K. A., Tsuji K., Enzinger R. M. Potentiating effect of calcium gluconate on the Limulus amebocyte lysate (LAL) gelation-endpoint assay for endotoxin. J Parenter Sci Technol. 1981 Sep-Oct;35(5):242–247. [PubMed] [Google Scholar]
- Takagi T., Hokama Y., Morita T., Iwanaga S., Nakamura S., Niwa M. Amino acid sequence studies on horseshoe crab (Tachypleus tridentatus) coagulogen and the mechanism of gel formation. Prog Clin Biol Res. 1979;29:169–184. [PubMed] [Google Scholar]
- Tsuji K., Steindler K. A. Use of magnesium to increase sensitivity of Limulus amoebocyte lysate for detection of endotoxin. Appl Environ Microbiol. 1983 Apr;45(4):1342–1350. doi: 10.1128/aem.45.4.1342-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



