Abstract
We compared the potency of SMP-601, a novel carbapenem, with that of vancomycin in a murine model of hematogenous bronchopneumonia infection caused by methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-intermediate S. aureus (VISA). The MICs of SMP-601 and vancomycin against MRSA were 2 and 1 μg/ml, respectively, while those against VISA were 2 and 8 μg/ml, respectively. Treatment with SMP-601 resulted in a significant decrease in the number of viable bacteria in the MRSA infection model (control, 100 mg/kg vancomycin, and 100 mg/kg SMP-601, 8.42 ± 0.50, 5.29 ± 0.71, and 5.50 ± 0.58 log CFU/lung, respectively,) and in the VISA infection model (control, 100 mg/kg vancomycin, and 100 mg/kg SMP-601, 9.64 ± 0.63, 8.72 ± 0.45, 7.42 ± 0.14 log CFU/lung) (mean ± standard error of the mean). The survival rate in the VISA infection model treated with SMP-601 (70%) was significantly higher than those in the other two groups (20% for vancomycin and 0% for control; P < 0.05). Histopathological examination revealed that inflammatory changes in the SMP-601-treated group were less marked than in the other two groups. The results of pharmacokinetic-pharmacodynamic analysis supported the results of the bacteriological, histopathological and survival studies. Our results demonstrate the potency of SMP-601 against MRSA and VISA in murine hematogenous pulmonary infection.
Methicillin-resistant Staphylococcus aureus (MRSA) was first identified in the 1960s, and the acquisition of genes encoding an additional penicillin-binding protein, PBP2a, which has low affinity to β-lactams, resulted in the emergence of MRSA in many health care institutions around the world. Currently, glycopeptides such as vancomycin and teicoplanin still provide effective therapy against most strains of MRSA. However, the first MRSA strain to acquire resistance to vancomycin was isolated from a Japanese patient in 1996 (4). Subsequent isolation of several vancomycin-intermediate S. aureus (VISA) strains from the United States, France, Korea, South Africa, and Brazil has confirmed that emergence of vancomycin resistance in S. aureus is a global issue (9). Subsequently, novel agents against MRSA and VISA, including linezolid, daptomycin, and quinupristin-dalfopristin, were developed. Ueda et al. (11) reported that SMP-601, a new carbapenem antibacterial agent, showed excellent antibacterial activity against clinical isolates of drug-resistant bacteria such as MRSA, methicillin-resistant Staphylococcus epidermidis, penicillin-resistant Streptococcus pneumoniae, ampicillin-resistant Haemophilus influenzae, and vancomycin-resistant Enterococcus faecium (VRE). The effectiveness of SMP-601 against systemic S. aureus infection in immunosuppressed mice reflected its in vitro activity (11).
In order to evaluate the efficacy of antibacterial agents and the pathogenesis of blood-borne staphylococcal pneumonia, we previously established a murine model of pulmonary infection with S. aureus by intravenous injection of bacteria enmeshed in agar beads (6, 8, 12-14). The aim of the present study was to evaluate the activity of SMP-601 against pulmonary infection caused by MRSA and VISA, in comparison with vancomycin, using the above model. We evaluated the antibacterial and histopathological effects, as well as the pharmacokinetics, of SMP-601 and vancomycin in MRSA and VISA infection models and conducted a survival study in a VISA infection model.
MATERIALS AND METHODS
Laboratory animals.
Six-week-old, male, ddY, specific-pathogen-free mice (25 to 30 g body weight) were purchased from Shizuoka Agricultural Cooperative Association Laboratory Animals (Shizuoka, Japan). All animals were housed in a pathogen-free environment and received sterile food and water in the Laboratory Animal Centre for Biomedical Science at Nagasaki University. The Ethics Review Committee for Animal Experimentation approved all experimental protocols in advance at our institution.
Bacterial strain.
Two strains of S. aureus were examined. The NUMR101 strain (MRSA) was isolated at Nagasaki University Hospital from the blood of an inpatient with pneumonia. Mu50, a VISA strain, was kindly provided by K. Hiramatsu (Juntendo University, Tokyo, Japan). Bacteria were stored at −80°C in Microbank (Pro-Lab Diagnostics, Ontario, Canada) until use.
Determination of MICs.
SMP-601 (Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan) and vancomycin (Shionogi Pharmaceutical Co., Osaka, Japan) were dissolved in sterile water immediately before use. The MIC of each agent was determined by the broth microdilution technique using Mueller-Hinton II broth, with an inoculum size of 5 × 105 CFU/ml. MIC was defined as the lowest concentration of the test agent that inhibited viable growth of bacteria after 18 h of incubation at 37°C. The MICs of SMP-601 and vancomycin for NUMR101 were 2 and 1 μg/ml, and those for Mu50 were 2 and 8 μg/ml, respectively.
Inoculum.
The method of inoculation was described previously (6, 8, 12-14). Briefly, for the MRSA study, NUMR101 was cultured overnight on Mueller-Hinton II agar plates at 37°C. Bacteria were suspended in endotoxin-free sterile saline and harvested by centrifugation (3,000 rpm, 4°C, 10 min). Organisms were resuspended in cold sterile saline and diluted to between 2 × 109 and 4 × 109 CFU/ml in the MRSA study and 4 × 109 and 6 × 109 CFU/ml or 2 × 1011 to 4 × 1011 CFU/ml in the VISA study, as estimated by turbidimetry. The suspension was warmed to 45°C, and 10 ml of the suspension was then mixed with 10 ml of 4% (wt/vol) molten Noble agar (Difco Laboratories, Detroit, MI) at 45°C. The agar-bacterium suspension (1.0 ml) was placed into a 1.0-ml syringe, and the suspension was rapidly injected via a 26-gauge needle into 49 ml of rapidly stirred ice-cooled sterile saline. This resulted in solidification of the agar droplets into beads of approximately 200 μm in diameter. The final concentration of agar was 0.04% (wt/vol), and the final number of bacteria was 2 × 107 to 4 × 107 CFU/ml. For the VISA study, the final number of Mu50 cells was 4 × 107 to 6 × 107 CFU/ml for bacteriological and histopathological studies or 2 × 109 to 4 × 109 CFU/ml for survival studies.
Animal model of hematogenous pneumonia.
We injected 0.25 ml of the suspension containing agar beads with bacteria into the tail vein of each mouse (10 ml/kg of body weight). The method used for inducing infection has been described in detail elsewhere (8, 12). Treatment commenced a day after inoculation by intraperitoneal (i.p.) administration of the test agent. For the MRSA study, 50 animals were allocated into five treatment groups: SMP-601 (10-mg/kg/dose and 100-mg/kg/dose), vancomycin (10-mg/kg/dose and 100-mg/kg/dose), or control (n = 10 in each group). The 10-mg/kg/doses of both drugs in this murine model were equivalent to the clinical dose of each drug in humans (0.5 to 1 g) in regard to the dose/body weight ratio. On the other hand, the 100-mg/kg/doses of both drugs in this model were equivalent to the clinical dose of each drug in humans (1 g) in regard to the area under the concentration-time curve (AUC) of total drug in serum. Each drug was administered twice daily (b.i.d.) for 7 days. For the VISA study, mice were pretreated with cyclophosphamide (100 mg/kg) at 1 day and 3 days before inoculation of Mu50, because of the low virulence of Mu50 against ddY mice, and were then treated with SMP-601 (100-mg/kg/dose b.i.d.), vancomycin (100-mg/kg/dose b.i.d.), or control (n = 10 in each group) for 3 days for bacteriological and histopathological studies or for 10 days for survival studies.
Bacteriological, survival, and histopathological examinations.
Each group of animals was sacrificed at specific time intervals by cervical dislocation. After exsanguination, lungs were dissected and removed under aseptic conditions. Organs used for bacteriological analysis were homogenized and cultured quantitatively by serial dilutions on Mueller-Hinton II agar plates. Lung tissue for histological examination was fixed in 10% buffered formalin and stained with hematoxylin-eosin. Specimens were examined under a light microscope, and total abscesses in that single slice were counted. Lung area was calculated using cross-section paper.
Lung tissue and serum concentrations of drugs.
Mice infected with MRSA NUMR101 were used for pharmacokinetic study. After 1 day of infection, 100 mg/kg of SMP-601 or vancomycin was administered i.p. Mice were sacrificed by cervical dislocation at 5, 15, 30, 60, 90, or 120 min after the completion of drug administration. Serum was separated after the blood had clotted. Three animals were used for each group at each time point. Each group of animals was sacrificed at specific time intervals by cervical dislocation. After exsanguination, lungs were dissected and removed under aseptic conditions and homogenized with saline. These samples (serum and lung homogenate) were immediately frozen and stored at −80°C until the assay was performed.
Concentrations of SMP-601 in lung homogenate and serum were measured by the paper disk bioassay method with Bacillus subtilis ATCC 6633 (limit of detection, 0.07 μg/ml in serum and 0.12 μg/ml in lung homogenate). Standard curves were made by the standard solutions of SMP-601 with pooled murine serum and lung homogenate. The disk diffusion bioassay was performed in triplicate with 50 μl of serum and lung homogenate from SMP-601-administered mice.
Concentrations of vancomycin were measured by a fluorescence polarization immunoassay with a an Abbott TDx-vancomycin (Abbott) kit and TDx analyzer (Abbott) (limit of detection, 2 μg/ml in serum and in lung homogenate). Standard curves were made by the standard solutions of vancomycin with pooled murine serum and lung homogenate. The fluorescence polarization immunoassay was performed with serum and lung homogenate from vancomycin-administered mice.
Standard curves were generated by linear regression. Samples with unknown concentrations of drugs in serum and lung homogenate were calculated from the equation of the line. The pharmacokinetic parameters were calculated according to moment analysis.
Statistical analysis.
Bacteriological data are expressed as means ± standard errors of the mean. Survival data are expressed by Kaplan-Meier curves. Differences between groups were examined for statistical significance by unpaired t test. A P value of <0.05 denoted the presence of a statistically significant difference.
RESULTS
Therapeutic effects of antibacterial agents.
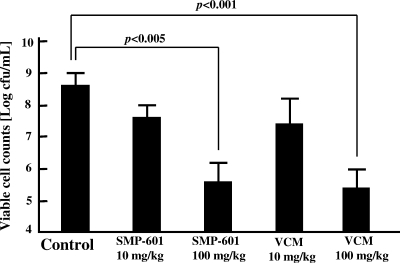
In the MRSA study, the number of bacteria in the lungs of the control group (untreated group) was 8.42 ± 0.50 log CFU/ml. In contrast, the numbers in the 100-mg/kg SMP-601 and 100-mg/kg vancomycin groups were 5.50 ± 0.58 and 5.29 ± 0.71 log CFU/ml, respectively. Thus, administration of these agents resulted in significant decreases in the number of viable MRSA cells compared with controls (P < 0.01). There were no significant differences between 100 mg/kg SMP-601 and 100 mg/kg vancomycin, while the 10-mg/kg SMP-601 and 10-mg/kg vancomycin groups did not significantly differ from the control group (Fig. 1).
FIG. 1.
Effects of SMP-601 and vancomycin (VCM) on the number of bacteria in lungs with MRSA hematogenous pulmonary infection. Mice were treated with each agent twice daily for 7 days after infection with NUMR101 (n = 10 in each group).
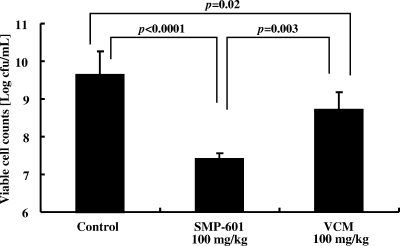
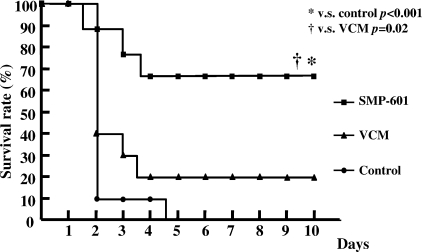
In the VISA study, mice were pretreated with cyclophosphamide (100 mg/kg) at 1 day and 3 days before inoculation because VISA Mu50 had lower pathogenicity than MRSA NUMR101. First, mice were infected with VISA at the same bacterial burden with MRSA (2 × 107 to 4 × 107 CFU/ml) and treated for 7 days. However, there were very few bacteria in lungs in controls and no difference between control and treated groups (data not shown). Therefore, we infected mice with VISA at 4 × 107 to 6 × 107 CFU/ml and treated them for 3 days before the control group died. Administration of 100 mg/kg SMP-601 resulted in a significant decrease in the number of viable VISA cells (7.42 ± 0.14 log CFU/ml) compared with the control (9.64 ± 0.63 log CFU/ml; P < 0.0001) and 100-mg/kg vancomycin groups (8.72 ± 0.45 log CFU/ml; P = 0.003) (Fig. 2). In the survival study, VISA cells were inoculated at 100-fold the concentration in the bacteriological examination. The survival rate at 10 days postinoculation was significantly higher in the 100-mg/kg SMP-601 treatment group (70% survival) than those in the 100-mg/kg vancomycin treatment group (20%; P = 0.02) and control groups (all dead within 4 days; P < 0.001) (Fig. 3).
FIG. 2.
Effects of SMP-601 and vancomycin (VCM) on the number of bacteria in lungs with VISA hematogenous pulmonary infection. Mice were treated with each agent twice daily for 3 days after infection with Mu50 (n = 10 in each group).
FIG. 3.
Effects of SMP-601 and vancomycin (VCM) on survival rate with VISA hematogenous pulmonary infection. Mice were treated with each agent (100 mg/kg/dose) twice daily after infection with Mu50. The survival rate was determined daily for10 days (n = 10 in each group).
Histopathological examination.
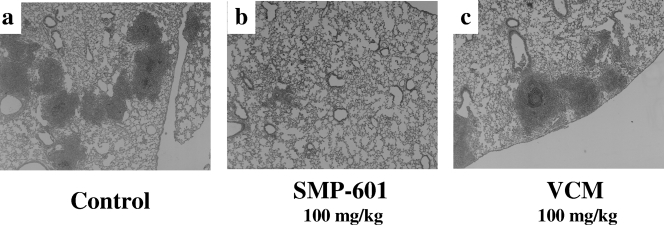
Microscopic examination of lung specimens from mice infected with Mu50 at 3 days after treatment revealed lung abscesses consisting of a central bacterial colony with infiltrating acute inflammatory cells (Fig. 4). The control (Fig. 4a) and vancomycin-treated (Fig. 4c) groups exhibited numerous abscesses and inflammation. In contrast, the SMP-601-treated group (Fig. 4b) exhibited fewer abscesses and less inflammation than the control and vancomycin-treated groups.
FIG. 4.
Histopathological examination of lung specimens from mice sacrificed 3 days after infection with VISA Mu50. Each specimen exhibited typical features of bronchopneumonia, with accumulation of neutrophils inside the bronchial lumen, infiltration of acute inflammatory cells, and exudates in the alveolar spaces (hematoxylin and eosin stain; original magnification, ×50). (a) Control mice. (b) SMP-601-treated mice. (c) Vancomycin (VCM)-treated mice. Note that the severity of inflammatory process is lower in the SMP-601-treated group than in the other groups.
When counting abscesses in whole lung specimens, significant decreases in the SMP-601-treated group were seen compared with the control and vancomycin-treated groups (Fig. 5a). The number of lung abscesses per mm2 showed a similar trend (Fig. 5b).
FIG. 5.
Abscesses in whole lungs (a) and per lung area (b) in the VISA Mu50 infection model. Specimens were examined under a microscope, and lung area was determined using cross-section paper (n = 3 in each group). VCM, vancomycin.
Pharmacokinetics of SMP-601 and vancomycin in serum and lung tissue of murine MRSA infection model.
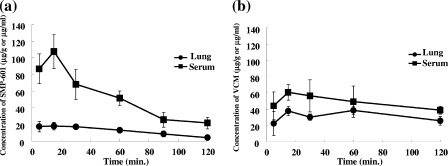
The pharmacokinetics of SMP-601 (100 mg/kg, i.p.) and vancomycin (100 mg/kg, i.p.) in serum and lung tissue of the MRSA NUMR101 infection model were investigated. Both drugs, especially vancomycin, showed good penetration from bloodstream into lung tissue in this infection model (Fig. 6 and Table 1). The AUC from 0 h to ∞ (AUC0-∞) values for vancomycin (total drug concentration in serum, 261 μg · h/ml; free drug concentration in serum, 196 μg · h/ml; total drug concentration in lung tissue, 127 μg · h/g) were remarkably larger than those of SMP-601 (total drug concentration in serum, 127 μg · h/ml; free drug concentration in serum, 30.6 μg · h/ml; total drug concentration in lung tissue, 28.4 μg · h/g) in this model. This advantage of vancomycin in AUC was mainly due to the longer half-life (t1/2) and lower serum protein binding rate of the drug compared with those of SMP-601.
FIG. 6.
Pharmacokinetics of SMP-601 and vancomycin (VCM) in serum and lung tissue of the model of murine MRSA NUMR101 pulmonary infection. Mice were treated with SMP-601 (a) or vancomycin (b) at 100 mg/kg once after bacterial inoculation (n = 3 in each group at each time point).
TABLE 1.
Total and free drug pharmacokinetic parameters of SMP-601 and vancomycin in serum and lung tissue of the murine MRSA pulmonary infection modela
| Antibiotic (100 mg/kg) and sampleb | AUC0-∞ (μg · h/ml) | Cmax (μg/ml) | t1/2 (h) |
|---|---|---|---|
| SMP-601 | |||
| Serum | |||
| Total | 127 | 107.53 | 0.84 |
| Freec | 30.6 | 25.81 | 0.84 |
| Lung (total) | 28.4e | 18.00f | 0.61 |
| Vancomycin | |||
| Serum | |||
| Total | 261 | 60.80 | 2.95 |
| Freed | 196 | 45.60 | 2.95 |
| Lung (total) | 127e | 38.50f | 1.72 |
Three mice were used in each group at each time point. Pharmacokinetic parameters were calculated from the arithmetic means of concentrations in serum and lung homogenate of three animals in each group according to the moment analysis. Cmax, maximum concentration of drug in serum.
Each agent (100 mg/kg) was administered i.p. 1 day after infection.
The protein binding rate of SMP-601 in murine serum was 76% (3).
The protein binding rate of vancomycin in murine serum was 25% (7).
AUC0-∞ in lung tissue measured as μg · h/g of lung tissue.
Cmax in lung tissue measured as μg/g of lung tissue.
PK-PD analysis of SMP-601 and vancomycin in the murine MRSA pulmonary infection model.
Table 2 shows the most important pharmacokinetic-pharmacodynamic (PK-PD) parameter of each drug (for SMP-601, the percentage of time the serum drug concentration is greater than the MIC [%T > MIC]; and for vancomycin, the AUC0-24/MIC) in the serum and lung tissues of the murine MRSA infection model. The MICs of SMP-601 and vancomycin against MRSA NUMR101 were 2 μg/ml and 1 μg/ml, respectively. In the 10-mg/kg b.i.d. model, which was equivalent to the clinical dose of each drug in humans (0.5 to 1 g/dose) in regard to the dose/body weight ratio, %T > MIC of SMP-601 (0 to 16.9%) and AUC0-24/MIC of vancomycin (25.4 to 52.2) were relatively small. On the other hand, in the 100-mg/kg b.i.d. model, which was equivalent to the clinical dose of each drug in humans (1 g) with regard to the AUC of total drug in serum, the %T > MIC of SMP-601 (20.3 to 28.2%) and AUC0-24/MIC of vancomycin (254 to 522) were high.
TABLE 2.
PK-PD parameters of SMP-601 and vancomycin in the murine MRSA pulmonary infection modela
PK-PD analysis of SMP-601 and vancomycin in the murine VISA pulmonary infection model.
Table 3 shows the most important PK-PD parameter of each drug in the serum and lung tissues of the murine VISA infection model. The MICs of SMP-601 and vancomycin against VISA Mu50 were 2 μg/ml and 8 μg/ml, respectively. In the 100-mg/kg b.i.d. model, %T > MIC of SMP-601 were high (20.3 to 28.2%), but the AUC0-24/MIC of vancomycin were low (31.8 to 65.3).
TABLE 3.
PK-PD parameters of SMP-601 and vancomycin in the murine VISA pulmonary infection modela
DISCUSSION
In the present study, we compared the efficacies of SMP-601 and vancomycin in MRSA and VISA lung infection models. In the MRSA study, SMP-601 showed beneficial efficacy on bacteriological examination, but the effectiveness was the same as that of vancomycin. In the VISA study, while vancomycin had little effect on bacteriological analysis and survival rate, SMP-601 protected mice against fatal pneumonia and resulted in a significant reduction in mortality. We previously showed that linezolid, the first oxazolidinone agent, DQ-113 and DX-619, novel quinolone agents, and quinupristin-dalfopristin, a complex of streptogramin A and B, are also effective against MRSA and VISA in this model, while vancomycin repeatedly shows little effect (6, 12-14).
The first report of a clinical S. aureus isolate that demonstrated reduced susceptibility to vancomycin in 1997 has been followed by multiple reports of additional isolates in all parts of the world. Recently, a third clinical isolate of vancomycin-resistant S. aureus (VRSA) was reported. VRSA evolves by acquisition of the vanA gene from enterococci, which are significant reservoirs of resistance genes among gram-positive bacterial pathogens (1). At present, there have been three independent cases of VRSA in the United States; in two of them, an association with vanA-containing enterococci at the same site in the affected patients could be established (2).
The new oxazolidinone antimicrobial linezolid has been approved for the treatment of infections caused by various gram-positive bacteria, including MRSA and VRE. However, one MRSA strain resistant to linezolid was isolated from a patient treated with this agent for dialysis-associated peritonitis (10). These reports illustrate the necessity of preparing optimal antimicrobial agents against VISA. According to in vitro data (11) and our results, SMP-601, a novel parenteral carbapenem, is a potentially reliable candidate.
β-Lactam agents inhibit synthesis of the bacterial cell wall by binding to penicillin-binding protein (PBP). Carbapenems, which are a type of β-lactam agent, have broad antimicrobial activity and high resistance to β-lactamase and are thus very useful for severe bacterial infections. SMP-601 has excellent antibacterial activity against drug-resistant bacteria, including MRSA, methicillin-resistant E. faecium, penicillin-resistant S. pneumoniae, VRE, and ampicillin-resistant Haemophilus influenzae. SMP-601 has a notably high affinity for PBP2a of MRSA, and this contributes to the strong anti-MRSA activity. The MIC90 for MRSA was comparable to those of linezolid and vancomycin.
Previous reports indicated that the most important PK-PD parameters of SMP-601 and vancomycin were %T > MIC and AUC0-24/MIC in serum, respectively (3, 5).
In the model with 10-mg/kg b.i.d. treatment against MRSA infection, the %T > MIC of SMP-601 in serum and lung tissue were 2.9% (free-drug in serum) and 0% (total drug in lung tissue), respectively, and the AUC0-24/MIC of vancomycin were 39.2 (free drug in serum) and 25.4 (total drug in lung tissue), respectively. These values of both drugs were considered to be insufficient for in vivo therapeutic efficacy of each agent. Eguchi et al. reported that SMP-601 required %T > MIC greater than 23% (free drug in serum) for in vivo efficacy against an MRSA thigh infection model (3).
Hyatt et al. reported that vancomycin needed an AUC0-24/MIC greater than 125 for satisfactory outcome in a clinical study (5).
In the model of 100-mg/kg b.i.d. treatment against MRSA infection and in the model of VISA infection, the %T > MIC of SMP-601 in serum and lung tissue were 23.6% (free drug in serum) and 20.3% (total drug in lung tissue), respectively. These values of %T > MIC were considered to be sufficient for antibacterial effect of SMP-601 in an animal infection model (3).
In the model of 100-mg/kg b.i.d. treatment with vancomycin against MRSA infection, the AUC0-24/MIC of vancomycin in serum and lung-tissue were sufficiently high, 392 (free-drug in serum) and 254 (total drug in lung tissue), respectively. On the other hand, in the model of 100-mg/kg b.i.d. treatment with vancomycin against VISA infection, the AUC0-24/MIC of vancomycin in serum and lung tissue were insufficient for therapeutic effect: 49.0 (free drug in serum) and 31.8 (total drug in lung tissue), respectively.
These PK-PD data supported the results of the bacteriological, histopathological, and survival studies. As shown in Table 1, in these murine infection models, pharmacokinetic conditions of vancomycin in serum and lung tissue were apparently superior to those of SMP-601 at the same dose. Therefore, the excellent therapeutic effect of SMP-601 against VISA infection model observed in this study was mainly caused by the strong anti-VISA activity of this agent.
Carbapenem agents have a very broad antibacterial spectrum, but existing carbapenems have no activity against MRSA and VISA. On the other hand, the novel carbapenem agent SMP-601 was found to be effective against MRSA, VISA, and other clinical isolates of drug-resistant bacteria. Furthermore, in the present study, we demonstrated in vivo efficacy against VISA. Thus, SMP-601 is a good candidate for MRSA, VISA, multibacterial infection, and early infection in which the susceptibility of the bacteria is unknown.
In conclusion, SMP-601 is a novel, promising carbapenem agent. This agent clearly reduced the number of bacteria in MRSA and VISA hematogenous infection mouse models and significantly improved the survival rate of mice infected with VISA compared with vancomycin. SMP-601 is thus considered to be clinically effective against MRSA and VISA, and the pharmacokinetic profiles are also promising.
Acknowledgments
We thank K. Kanazawa (Dainippon Sumitomo Co., Tokyo, Japan) for assistance with pharmacokinetic analyses.
R. Kihara and K. Yanagihara contributed equally to this work.
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. MMWR Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 2.Chang, S., D. M. Sievert, J. C. Hageman, et al. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi, K., and K. Kanazawa. 2007. In vivo pharmacodynamic activity of SMP-601 (PZ-601) in a new VRE thigh infection model using carrageenan-treated mice, p. 230. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Hiramatsu, K., H. Hanaki, T. Ino, et al. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 5.Hyatt, J. M., P. S. McKinnon, G. S. Zimmer, et al. 1995. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin. Pharmacokinet. 28:143-160. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko, Y., K. Yanagihara, Y. Miyazaki, K. Tsukamoto, Y. Hirakata, K. Tamano, J.-I. Kadota, T. Tashiro, I. Murata, and S. Kohno. 2003. Effects of DQ-113, a new quinolone, against methicillin- and vancomycin-resistant Staphylococcus aureus-caused hematogenous pulmonary infections in mice. Antimicrob. Agents Chemother. 47:3694-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudsen, J. D., K. Fuursted, F. Espersen, and N. Frimodt-Møller. 1997. Activities of vancomycin and teicoplanin against penicillin-resistant pneumococci in vitro and in vivo and correlation to pharmacokinetic parameters in the mouse peritonitis model. Antimicrob. Agents Chemother. 41:1910-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawai, T., K. Tomono, K. Yanagihara, Y. Yamamoto, M. Kaku, Y. Hirakata, H. Koga, T. Tashiro, and S. Kohno. 1997. Role of coagulase in a murine model of hematogenous pulmonary infection induced by intravenous injection of Staphylococcus aureus enmeshed in agar beads. Infect. Immun. 65:466-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith, T. L., M. L. Pearson, K. R. Wilcox, et al. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 10.Tsiodras, S., H. S. Gold, G. Sakoulas, et al. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 11.Ueda, Y., K. Kanazawa, K. Eguchi, K. Takemoto, Y. Eriguchi, and M. Sunagawa. 2005. In vitro and in vivo antibacterial activities of SM-216601, a new broad-spectrum parenteral carbapenem. Antimicrob. Agents Chemother. 49:4185-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagihara, K., Y. Kaneko, T. Sawai, Y. Miyazaki, K. Tsukamoto, Y. Hirakata, K. Tomono, J.-I. Kadota, T. Tashiro, I. Murata, and S. Kohno. 2002. Efficacy of linezolid against methicillin-resistant or vancomycin-insensitive Staphylococcus aureus in a model of hematogenous pulmonary infection. Antimicrob. Agents Chemother. 46:3288-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagihara, K., M. Okada, and Y. Fukuda, et al. 2004. Efficacy of quinupristin-dalfopristin against methicillin-resistant Staphylococcus aureus and vancomycin-insensitive S. aureus in a model of hematogenous pulmonary infection. Chemotherapy 50:260-264. [DOI] [PubMed] [Google Scholar]
- 14.Yanagihara, K., M. Seki, K. Izumikawa, et al. 2006. Potency of DX-619, a novel des-F (6)-quinolone, in hematogenous murine bronchopneumonia caused by methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Int. J. Antimicrob. Agents 28:212-216. [DOI] [PubMed] [Google Scholar]