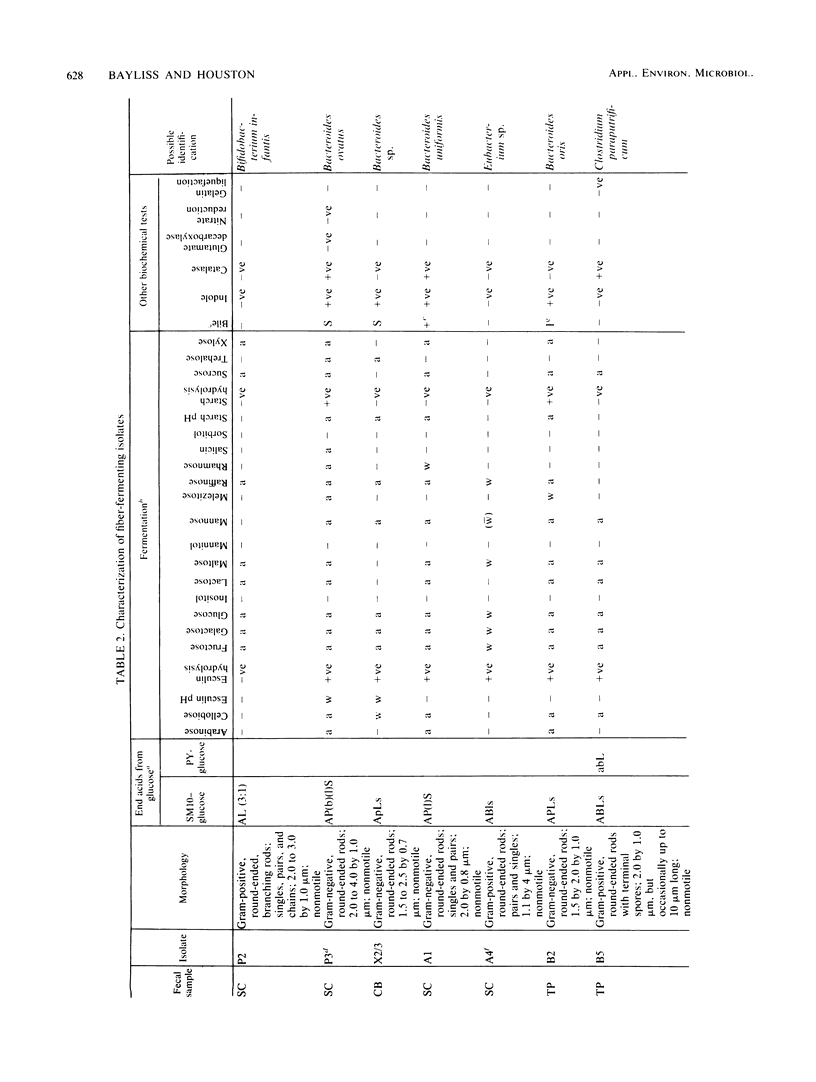
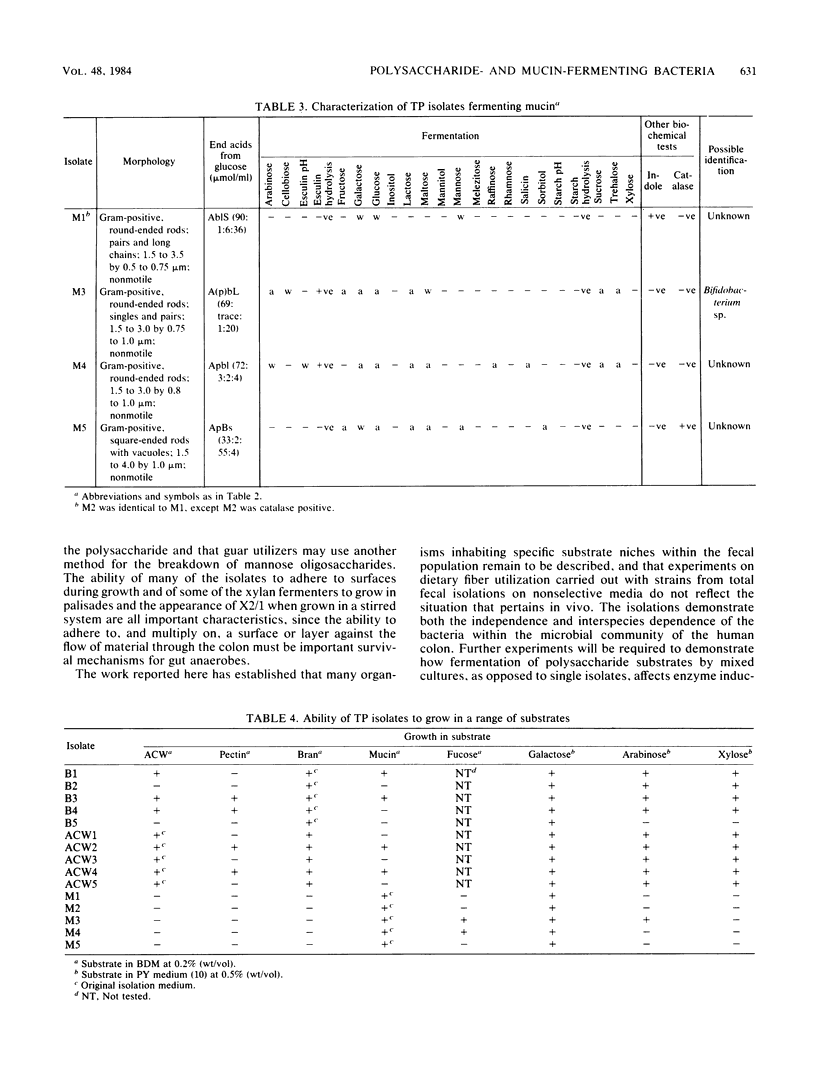
Abstract
Organisms able to grow on arabinogalactan, pectin, xylan, wheat bran, guar, apple cell walls, and mucin were isolated by enrichment from human feces. The number of polysaccharide fermenters and the properties of the predominant bacteria varied between subjects. The ability to use one polysaccharide was not related to the ability to use others. Some organisms (e.g., Bacteroides spp.) isolated on other substrates also utilized mucin, but were not isolated in the mucin enrichment. The mucin fermenters isolated by enrichment had a very restricted ability to utilize complex polysaccharides and their constituent monosaccharides, suggesting that the presence of plant polysaccharides in the human colon is unlikely to prevent the use of colonic mucin as an energy source by bacteria. Characterization with a range of biochemical tests showed that many of the isolates, but especially the mucin fermenters, did not resemble organisms described previously.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Impey C. S., Stevens B. J., Peel J. L. Streptococcus pleomorphus sp.nov.: an anaerobic streptococcus isolated mainly from the caeca of birds. J Gen Microbiol. 1977 Sep;102(1):45–53. doi: 10.1099/00221287-102-1-45. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Impey C. S. The occurence and properties of uric acid decomposing anaerobic bacteria in the avian caecum. J Appl Bacteriol. 1974 Sep;37(3):393–409. doi: 10.1111/j.1365-2672.1974.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Croucher S. C., Houston A. P., Bayliss C. E., Turner R. J. Bacterial populations associated with different regions of the human colon wall. Appl Environ Microbiol. 1983 Mar;45(3):1025–1033. doi: 10.1128/aem.45.3.1025-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Branch W., Jenkins D. J., Southgate D. A., Houston H., James W. P. Colonic response to dietary fibre from carrot, cabbage, apple, bran. Lancet. 1978 Jan 7;1(8054):5–9. doi: 10.1016/s0140-6736(78)90357-4. [DOI] [PubMed] [Google Scholar]
- Dekker J., Palmer J. K. Enzymatic degradation of the plant cell wall by a Bacteroides of human fecal origin. J Agric Food Chem. 1981 May-Jun;29(3):480–484. doi: 10.1021/jf00105a010. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbal I., Kells D. I., Forstner G., Forstner J. Human intestinal goblet cell mucin. Can J Biochem. 1976 Aug;54(8):707–716. doi: 10.1139/o76-102. [DOI] [PubMed] [Google Scholar]
- Linn S., Chan T., Lipeski L., Salyers A. A. Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J Bacteriol. 1983 Nov;156(2):859–866. doi: 10.1128/jb.156.2.859-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. S., Hoskins L. C. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a "most probable number" method. Gastroenterology. 1981 Oct;81(4):759–765. [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvendran R. R., March J. F., Ring S. G. Determination of aldoses and uronic acid content of vegetable fiber. Anal Biochem. 1979 Jul 15;96(2):282–292. doi: 10.1016/0003-2697(79)90583-9. [DOI] [PubMed] [Google Scholar]
- Stephen A. M., Cummings J. H. The microbial contribution to human faecal mass. J Med Microbiol. 1980 Feb;13(1):45–56. doi: 10.1099/00222615-13-1-45. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P., Holdeman L. V., Moore W. E. Isolation of ureolytic Peptostreptococcus productus from feces using defined medium; failure of common urease tests. Appl Microbiol. 1974 Oct;28(4):594–599. doi: 10.1128/am.28.4.594-599.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


