Abstract
Microsporidia are eukaryotic, obligate, intracellular protists that are emerging pathogens in immunocompromised hosts, including AIDS patients and organ transplant recipients. The efficacy of gamma interferon (IFN-γ) for the treatment of microsporidiosis caused by Encephalitozoon cuniculi was studied by means of adoptive transfer and IFN-γ administration in SCID mice. While the adoptive transfer of CD4+ T cells from immunocompetent mice prolonged survival of SCID mice infected perorally with E. cuniculi, survival was not improved by adoptive transfer of CD4+ T lymphocytes from IFN-γ-deficient mice. The protective effect of IFN-γ was confirmed in cytokine therapy experiments in which SCID mice receiving IFN-γ survived significantly longer than mice receiving mock injections. The administration of serum containing specific antibodies against E. cuniculi was found to prolong the survival of concurrently IFN-γ-treated SCID mice. The data presented in this study suggest that IFN-γ is potentially useful as a cytokine therapy for microsporidiosis, especially in CD4+ T-cell-deficient patients.
Microsporidia are single-celled, obligately intracellular eukaryotic pathogens which cause microsporidiosis, an opportunistic infectious disease with a broad clinical spectrum of manifestations, including gastrointestinal, pulmonary, nasal, ocular, muscular, cerebral, and systemic infections, which may be lethal in immunodeficient hosts (12, 17, 25). Immune reconstitution resulting from antiretroviral therapy greatly reduces the occurrence of microsporidiosis in persons infected with human immunodeficiency virus (26), and currently available antimicrosporidial therapies have been shown to be useful in treatment, with albendazole, a benzimidazole that inhibits microtubule assembly, being effective against microsporidia of the genus Encephalitozoon, although less so against Enterocytozoon bieneusi (23), and fumagillin, an antibiotic produced by Aspergillus fumigatus, being effective against E. bieneusi (although toxic when administered systemically) (16). However, relapses of the disease are not uncommon, and so the improvement of therapeutic options is important for successful treatment. One potential therapeutic option involves the use of cytokines as an adjunct to conventional antimicrosporidial therapy.
Most what is known about the host immune response against microsporidia is based on the model infection of mice with Encephalitozoon cuniculi Levaditi, Nicolau et Schoen, 1923. It has been suggested that the protective immune response against microsporidia is mediated by cytotoxic CD8+ T lymphocytes (11). However, Braunfuchsova et al. (2) demonstrated that the significance of CD4+ and CD8+ T lymphocytes in the protection of mice against E. cuniculi infection differs depending on the route of infection. While CD8+ T lymphocytes are essential for protection after intraperitoneal (i.p.; artificial) infection, CD8+-T-lymphocyte-deficient mice are able to overcome the outcome of the disease following peroral (natural) infection. Our previous studies proposed that CD8+-T-lymphocyte-independent protection against the peroral route of infection is mediated by CD4+ T lymphocytes, producing gamma interferon (IFN-γ), and by B lymphocytes, producing specific antimicrosporidial antibody (19, 21). IFN-γ is essential for the survival of mice infected either i.p. or perorally (10, 20), apparently because of its ability to polarize adaptive immunity toward a Th1-type response, promoting the generation of CD8+ T-cell immunity. Moreover, it has been shown that IFN-γ-activated macrophages are able to kill microsporidia in vitro (6, 9).
In the present study, we examined the utility of IFN-γ, alone or in combination with specific anti-E. cuniculi antibody therapy, in combating peroral infection of SCID mice with the microsporidian E. cuniculi. This is the first report of the use of cytokine therapy of microsporidiosis in an immunodeficient host.
MATERIALS AND METHODS
Pathogens.
The spores of E. cuniculi strain EC2 were originally isolated from a dexamethasone-treated laboratory mouse (14) and were grown in vitro in green monkey kidney cells (Vero, line E6 originated from the Centers for Disease Control and Prevention tissue collection) maintained in RPMI 1640 medium (Sigma-Aldrich) supplemented with 2.5% heat-inactivated fetal bovine serum. Spores were purified from host cells by centrifugation over 50% Percoll (Sigma-Aldrich) at 1,100 × g for 30 min and washed three times in deionized water before storage in deionized water supplemented with antibiotics—100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2.5 μg of amphotericin/ml (all from Sigma-Aldrich)—at 4°C. The spores were washed in phosphate-buffered saline before use.
Mice.
BALB/c mice and SCID mice (strain C.B-17) on a BALB/c background were obtained from Charles River, Sulzfeld, Germany. Mice with a disrupted IFN-γ gene [IFN-γ knockout (KO) mice, strain C.1297S7(B6)-Ifngtm1Ts] on a BALB/c background were obtained from the Jackson Laboratory, Bar Harbor, ME. SCID mice and IFN-γ KO mice were housed in flexible film isolators (BEM, Znojmo, Czech Republic) with high-efficiency particulate air filters and supplied with sterilized diet and water ad libitum. BALB/c mice were caged in a mouse room with the temperature kept at 22°C, with a relative humidity of 65%. Mice aged 7 to 8 weeks at the time of infection were used throughout the experiments.
Isolation of CD4+ T lymphocytes.
Whole splenocytes from naive, wild-type BALB/c mice or from IFN-γ KO mice were obtained by mechanical disruption of the spleen and washed three times in RPMI 1640 medium. CD8+ T lymphocytes were depleted by complement-mediated lysis following labeling with anti-CD8 monoclonal antibody (MAb; rat anti-mouse MAb 2.43, kindly provided by Imtiaz Khan, Dartmouth Medical School), using complement obtained from guinea pig serum. CD4+ T lymphocytes were then isolated by using a Dynabead Mouse CD4 (L3T4) kit (Dynal Biotech ASA, Oslo, Norway) based on magnetic separation. The purity of CD4+ lymphocytes was analyzed by flow cytometry. Samples (0.5 × 106 cells) were incubated with CD4-specific MAb YTS 177.9 (conjugated with fluorescein isothiocyanate) and CD8-specific MAb KT15 (conjugated with phycoerythrin) obtained from Serotec (Oxford, United Kingdom) diluted 1:50 in 1% fetal bovine serum in phosphate-buffered saline. Labeled cell samples were analyzed on an Epics XL flow cytometer (Coulter) equipped with a 15-mW argon-ion laser with excitation capabilities at 488 nm. Ten thousand events were recorded. The labeled cell population was analyzed by using System II software (Coulter).
Adoptive transfer experiment 1.
Two groups of SCID mice were reconstituted with an i.p. dose of 2 × 106 CD4+ T cells from either BALB/c or IFN-γ KO mice in 0.5 ml of RPMI 1640 (eight and nine animals per group, respectively). Seven days after adoptive transfer, mice were infected per os (p.o.) with 107 E. cuniculi spores in 0.1 ml of deionized water by intragastric gavage. A third group composed of infected nonreconstituted SCID mice (eight animals) served as a control. Morbidity and mortality were recorded daily.
Adoptive transfer experiment 2.
Two groups of SCID mice (11 animals per group) were reconstituted with an i.p. dose of 2 × 106 CD4+ T cells from naive or immunized BALB/c mice in 0.5 ml of RPMI 1640. BALB/c donors were immunized i.p. with alive 107 E. cuniculi spores three times at 7-day intervals. The efficacy of immunization was assessed by enzyme-linked immunosorbent assay (ELISA) for anti-E. cuniculi antibodies. The third group of infected nonreconstituted SCID mice (10 animals) served as a control. P.o. infection was performed in the same way as in adoptive transfer experiment 1.
Murine recombinant IFN-γ expression and purification.
IFN-γ was amplified from cDNA generated from lipopolysaccharide-activated mouse splenocytes. Primers were designed based upon the sequence from GenBank (accession number NM_008337), excluding the signal peptide. The PCR product was cloned into an expression vector (pET100/D; Invitrogen) with a polyhistidine tag at the N-terminal region and expressed in E. coli strain BL21 (Invitrogen). Active recombinant IFN-γ was purified from the cytosolic fraction by using Ni2+ affinity chromatography under native conditions and eluted with 0.5 M imidazole solution. Purified protein was transferred into TBS buffer (20 mM Tris-HCl, 150 mM NaCl [pH 7.8]). Potential lipopolysaccharide contamination was removed by using EndoTrapRed (Cambrex). The level of endotoxin was determined by the LAL method (Cambrex). The activity of prepared IFN-γ was measured in an antiviral assay using L-929 cells infected with murine EMC virus. The 50% effective dose for this effect was comparable to that for IFN-γ obtained from R&D Systems, i.e., typically 0.5 ng/ml. IFN-γ was stored at 4°C until use.
Anti-E. cuniculi serum production.
BALB/c mice were immunized i.p. with 107 E. cuniculi spores three times at 15-day intervals. Mouse blood was drawn on the eighth day after the last immunization. After storage for 12 h at room temperature the blood was centrifuged at 160 × g for 10 min. The serum was obtained and stored at −25°C.
Recombinant IFN-γ therapy experiment 1.
Three groups of SCID mice (six animals per group) were randomly assigned to therapy groups. The groups consisted of (i) mice receiving 3 × 103 U of recombinant murine IFN-γ (R&D Systems) concurrently with anti-E. cuniculi serum (30 μl, ELISA titer of 1:25,600), diluted in 0.2 ml of sterile saline with 0.1% bovine serum albumin; (ii) mice receiving IFN-γ in diluent only; and (iii) mice receiving diluent only (the control group). Mice were given six therapy doses, with a dose administered i.p. every 3 days, beginning on the day of infection. P.o. infection was performed as described for adoptive transfer experiment 1.
Recombinant IFN-γ therapy experiment 2.
Three groups of SCID mice were randomly assigned to therapy groups. The groups included (i) mice receiving no therapy (four animals), (ii) mice receiving 1.5 × 104 U of IFN-γ (prepared in our laboratory) diluted in 0.2 ml of TBS buffer (six animals), (iii) and mice receiving diluent only (six animals). Mice were given 12 therapy doses, with a dose administered i.p. every 2 days, beginning on the day of infection. P.o. infection was performed as described for adoptive transfer experiment 1.
Effect of immunization on infection development.
Three groups of IFN-γ KO mice were used in this experiment. The first group (14 animals) was immunized i.p. with devitalized (heat-inactivated) E. cuniculi spores three times at 7-day intervals (107 inactivated spores in dose). The last dose was given to mice 5 days before experimental P.o. infection (performed as described for adoptive transfer experiment 1). The second group (14 animals) served as an infected, nonimmunized control. The third group (four animals) was immunized only, serving as a control for successful immunization. The presence of antibody in the sera of immunized mice was confirmed by ELISA.
Statistical analysis.
The differences in survival time among groups were analyzed by survival analysis (multiple-sample test) and a log-rank test for between-group analysis. The effect of immunization on the relative amounts of specific anti-E. cuniculi antibodies was analyzed by using the Student t test. Tests were performed by using Statistica 6.0 (StatSoft, Inc.).
RESULTS
Adoptive transfer experiment 1.
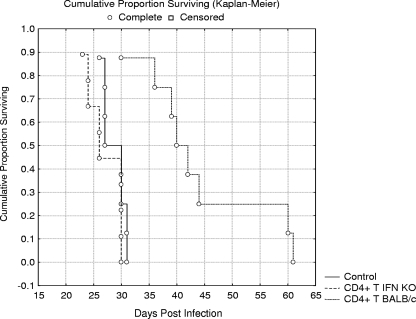
The role of IFN-γ produced by CD4+ T lymphocytes in the survival of perorally E. cuniculi-infected SCID mice was tested in this experiment. The protective effect conferred by the adoptive transfer of CD4+ T lymphocytes from wild-type BALB/c mice (able to produce IFN-γ) and CD4+ T lymphocytes from IFN-γ KO mice (unable to produce IFN-γ) is presented in Fig. 1. Reconstitution with CD4+ T lymphocytes from BALB/c mice resulted in a significant increase in survival (P < 0.01), with the mean survival time (MST) of animals in this group being 44.0 days. In contrast, the adoptive transfer of CD4+ T lymphocytes from IFN-γ KO mice did not have a statistically significant effect on survival, with an MST of 27.0 days, compared to an MST of 28.6 days for the infected control group of nonreconstituted SCID mice. The results represent pooled data from two experiments.
FIG. 1.
Survival of SCID mice reconstituted either with naive CD4+ T lymphocytes from BALB/c mice (CD4+ T BALB/c) or CD4+ T lymphocytes from IFN-γ KO mice (CD4+ T IFN KO) and infected with E. cuniculi p.o. A nonreconstituted group of p.o.-infected SCID mice served as a control (Control).
Adoptive transfer experiment 2.
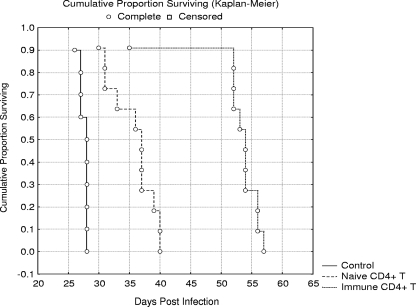
The effect of adoptive transfer of CD4+ T lymphocytes from immunocompetent BALB/c naive mice or from immunized BALB/c mice on SCID mouse survival was assessed. The influence of adoptive transfer on p.o. E. cuniculi infection development in SCID mice is shown in Fig. 2. The adoptive transfer of CD4+ T lymphocytes from naive BALB/c to SCID mice significantly (P < 0.01) extended their MST (35.5 days) compared to nonreconstituted infected SCID mice (MST = 27.5 days). Moreover, the p.o.-infected SCID mice reconstituted with CD4+ T lymphocytes from immunized BALB/c mice survived significantly (P < 0.01) longer (MST = 52.3 days) than other tested groups. The results represent pooled data from two experiments.
FIG. 2.
Survival of SCID mice reconstituted either with naive CD4+ T lymphocytes (Naive CD4+ T) or immune CD4+ T lymphocytes (Immune CD4+ T) from BALB/c mice and infected with E. cuniculi p.o. A nonreconstituted group of p.o.-infected SCID mice served as a control (Control).
Recombinant IFN-γ therapy experiment 1.
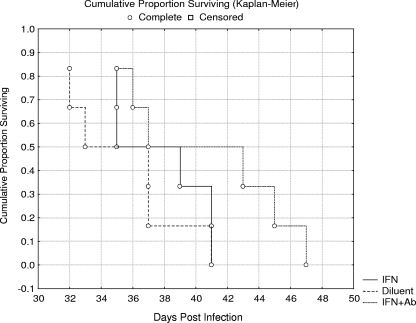
The parameter used to evaluate the efficacy of IFN-γ therapy in this experiment was the MST of experimentally treated groups of SCID mice p.o. infected with E. cuniculi (Fig. 3). The MST (37.7 days) of SCID mice receiving IFN-γ therapy (3 × 103 U of IFN-γ, six doses, every third day) was not statistically different from the group of SCID mice receiving diluent only (MST = 35.3 days). Concurrent therapy with IFN-γ and anti-E. cuniculi serum tended to prolong survival of treated mice (MST = 40.5 days), but this effect was not significant. The results represent pooled data from two experiments.
FIG. 3.
Efficacy of IFN-γ and specific antibody treatment on survival of SCID mice p.o. infected with E. cuniculi (experiment 1). IFN+Ab, concurrent application of IFN-γ and anti-E. cuniculi serum; IFN, application of IFN-γ (six doses of 3 × 103 U); Diluent, application of diluent only.
The effect of applications of anti-E. cuniculi serum was also tested as a part of this experiment. Seventeen doses of anti-E. cuniculi serum, administered every other day, had no effect on the survival time of p.o.-infected SCID mice. The MST (35.2 days) of mice receiving anti-E. cuniculi serum was not statistically different that of from mice receiving diluent only (MST = 34.4 days) or that of infected SCID mice only (MST = 34.5 days).
Recombinant IFN-γ therapy experiment 2.
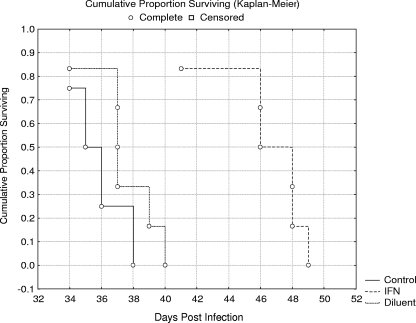
Treatment with larger and more frequent doses of IFN-γ was tested in this experiment (Fig. 4). In contrast to the previous experiment, the larger doses of IFN-γ (1.5 × 104 U of IFN-γ, 12 doses given every other day) significantly prolonged the survival of p.o.-infected SCID mice (P < 0.05; MST = 46.3 days). The MST (37.3 days) of SCID mice receiving diluent only was not statistically different from those infected and untreated (MST = 35.7).
FIG. 4.
Efficacy of IFN-γ treatment on the survival of SCID mice p.o. infected with E. cuniculi (experiment 2). IFN, application of IFN-γ (12 doses of 1.5 × 104 U); Diluent, application of diluent only; Control, no treatment.
Effect of immunization on infection development.
The role of specific anti-E. cuniculi antibodies on infection development in mice unable to produce IFN-γ was assessed. A statistically significant difference was not observed in MST between infected groups of immunized (MST = 36.1 days) and nonimmunized (MST = 34.6 days) IFN-γ KO mice. The results represent pooled data from three experiments. Screening for the presence of specific anti-E. cuniculi antibodies in the sera of immunized or nonimmunized IFN-γ KO mice at the time of infection revealed that immunization resulted in higher amounts of specific anti-E. cuniculi antibodies compared to nonimmunized animals (P < 0.001). Also, higher specific anti-E. cuniculi antibody production as a reaction on p.o. E. cuniculi infection on day 18 postinfection was observed in the sera of immunized IFN-γ KO mice than in nonimmunized mice (P < 0.01).
DISCUSSION
We tested the efficacy of IFN-γ and a specific anti-E. cuniculi antibody for the treatment of microsporidiosis caused by E. cuniculi in an immunocompromised host. In our previous studies we showed that CD8+-T-lymphocyte-independent protection against p.o.-acquired E. cuniculi infection is mediated by functional CD4+ T lymphocytes and by specific anti-E. cuniculi antibodies (19). Based on the essential role of IFN-γ for protection against lethal microsporidiosis (10, 20), we hypothesized that IFN-γ produced by CD4+ T lymphocytes could be the main mechanism that mediates partial protection of SCID mice reconstituted with CD4+ T cells.
This theory was tested in series of adoptive-transfer experiments. Adoptive transfer of CD4+ T lymphocytes from immunocompetent BALB/c mice prolonged the survival of infected SCID mice. In contrast, CD4+ T lymphocytes from IFN-γ KO mice on the same background did not affect survival time (Fig. 1). This result indicates that the partial protective effect of CD4+ T cells is mediated by IFN-γ production. Moreover, transfer of CD4+ T lymphocytes from immune animals increased the protective effect of these cells compared to the transfer of naive cells (Fig. 2). This effect could be explained by the fact that greater numbers of CD4+ T cells specifically recognizing microsporidial antigens were present in immune donors. In particular, CD4+ T lymphocytes specifically recognizing microsporidial antigens in a context with major histocompatibility complex class II molecules of antigen-presenting cells could be the main producers of IFN-γ in reconstituted hosts. There is evidence that IFN-γ is a potent activator of macrophages, resulting in the effective killing of phagocytosed microsporidial spores by the production of toxic oxygen metabolites (5, 6, 9). Another mechanism for the antimicrosporidial activity of IFN-γ may be its ability to enhance cytotoxic activity in natural killer cells.
The protective effect of IFN-γ was confirmed in therapy experiments. SCID mice receiving IFN-γ survived significantly longer than mice receiving diluent only (Fig. 4). The effect of IFN-γ therapy on SCID mice survival depended on the amount of administered cytokine: while 6 doses of 3 × 103 U did not affect the SCID mice survival, 12 doses of 1.5 × 104 U prolonged the survival of p.o.-infected mice. The cytokine therapy schedule used was designed based on the work of Clemons et al. (3), who used IFN-γ for the effective treatment of systemic cryptococcosis in SCID mice.
We have demonstrated that IFN-γ shows therapeutic efficacy against microsporidiosis in immunodeficient animals. Although cytokine application did not protect SCID mice from the eventual development of lethal disease, onset was delayed, indicating that IFN-γ may have therapeutic efficacy against microsporidiosis in immunocompromised patients. IFN-γ treatment could be particularly beneficial to AIDS patients at high risk of developing opportunistic infections, that is, individuals with significantly declined CD4+-T-lymphocyte counts (<100/mm3 of blood) but with functional CD8+ T cells.
It is well established that IFN-γ plays a mandatory role in acquired protective immunity to other opportunistic intracellular pathogens. For example, Kolls et al. (13) showed that upregulation of murine IFN-γ by gene transfer into lung tissue results in the clearance of Pneumocystis carinii from the lungs in the absence of CD4+ lymphocytes, with the resolution of infection associated with an increase in CD8+ T lymphocytes and natural killer cells in the lungs. Resistance to Cryptosporidium parvum infection in mice in the absence of adaptive immunity also appears to be IFN-γ dependent, since the use of an IFN-γ-neutralizing antibody in a murine model increased susceptibility to infection, and resistance to, and control of, infection was correlated with increased mucosal expression of IFN-γ (15). IFN-γ production was found to be essential for the prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice (24). Recently, IFN-γ therapy has been considered for the prophylactic treatment of latent tuberculosis infection and for shortening the protracted standard chemotherapy in immunodeficient patients, who often suffer from mycobacterial infections (18). Therefore, it seems conceivable that the application of recombinant IFN-γ could help CD4+-T-lymphocyte-deficient people (e.g., AIDS patients) to overcome a wide range of opportunistic infections.
On the other hand, it is important to emphasize that IFN-γ therapy cannot be used when targeted immunosuppression is part of the treatment (e.g., organ transplantation). In such cases, conventional chemotherapy (albendazole and fumagillin) must remain the first choice for treatment. Recently, therapeutic development studies have focused on compounds that target microsporidian polyamines (polyamine analogues), methionine aminopeptidase 2 (fumagillin analogues), chitin (nikkomycins), and topoisomerases (fluoroquinolones) (1, 4, 27).
The second aim of our study was to assess the role of specific antibodies during microsporidial infection. Our results demonstrate that these antibodies are protective only when administered concurrently with IFN-γ (Fig. 3) and, in accordance with this, immunization of IFN-γ KO mice had no convincing effect on survival. These data are in agreement with our previous study, in which specific anti-E. cuniculi antibodies contributed to the resistance mediated by CD4+ T lymphocytes (19). The binding of antibodies to antigens of microsporidial spores could enhance the phagocytosis (7). However, it has been proposed that phagocytosis of spores may actually facilitate cell infection, since the phagocytosis of spores has been reported to occur 10 times more frequently than the injection of sporoplasm via the polar tube (8). On the other hand, a microsporidial protein (EcMsAP) potentially involved in spore adherence to host cells has been recently described (22). Blocking of epitopes on this protein by specific antibodies might be part of the host strategy to control microsporidial infection.
The influence of specific antibodies on the MST of SCID mice was increased only with the concurrent application of IFN-γ in our experiment. This suggests that the effect of the antibodies was not simply an effect on invasion or growth but also required the IFN-γ-dependent activation of macrophages (or other IFN-γ-dependent mechanisms).
In conclusion, on the basis of the data presented here, we suggest that IFN-γ may be useful as a cytokine therapy for microsporidiosis, especially in CD4+-T-cell-deficient patients (e.g., AIDS patients). Humoral immunity clearly contributes to the response against microsporidial infection, but only in the presence of IFN-γ.
Acknowledgments
This study was supported by grant 524/03/D167 from the Grant Agency of the Czech Republic, by grant MSM-123100003 of the Ministry of Education, Youth, and Sports of the Czech Republic, and by the Institute of Parasitology, Biology Centre ASCR (Z60220518 and LC 60009).
All of the experimental procedures were done in accordance with the national law on the use of experimental animals, safety, and use of pathogenic agents.
We are grateful to Stephen Preston for his revision of the manuscript and to Jiří Jarkovský and Markéta Ondračková for help with statistical analysis.
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Bacchi, C. J., L. M. Weiss, S. Lane, B. Frydman, A. Valasinas, V. Reddy, J. S. Sun, L. J. Marton, I. A. Khan, M. Moretto, N. Yarlett, and M. Wittner. 2002. Novel synthetic polyamines are effective in the treatment of experimental microsporidiosis, an opportunistic AIDS-Associated infection. Antimicrob. Agents Chemother. 46:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunfuchsova, P., J. Salat, and J. Kopecky. 2002. Comparison of the significance of CD4+ and CD8+ T lymphocytes in the protection of mice against Encephalitozoon cuniculi infection. J. Parasitol. 88:797-799. [DOI] [PubMed] [Google Scholar]
- 3.Clemons, K. V., J. E. Lutz, and D. A. Stevens. 2000. Efficacy of recombinant gamma interferon for treatment of systemic cryptococcosis in SCID mice. Antimicrob. Agents Chemother. 45:486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Didier, E. S., J. A. Maddry, P. J. Brindley, M. E. Stovall, and P. J. Didier. 2005. Therapeutic strategies for human microsporidia infections. Expert Rev. Anti-Infect. Ther. 3:419-434. [DOI] [PubMed] [Google Scholar]
- 5.Didier, E. S., and J. A. Shadduck. 1994. IFN-γ and LPS induce murine macrophages to kill Encephalitozoon cuniculi in vitro. J. Eukaryot. Microbiol. 41:43. [PubMed] [Google Scholar]
- 6.Didier, E. S. 1995. Reactive nitrogen intermediates implicated in the inhibition of Encephalitozoon cuniculi (phylum Microspora) replication in murine peritoneal macrophages. Parasite Immunol. 17:405-412. [DOI] [PubMed] [Google Scholar]
- 7.Di Virgilio, F., B. C. Meyer, S. Greenberg, and S. C. Silverstein. 1988. Fc receptor-mediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J. Cell Biol. 106:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzen, C., A. Muller, P. Hartmann, and B. Salzberger. 2005. Cell invasion and intracellular fate of Encephalitozoon cuniculi (Microsporidia). Parasitology 130:285-292. [DOI] [PubMed] [Google Scholar]
- 9.Jelinek, J., J. Salat, B. Sak, and J. Kopecky. 2007. Effects of interferon gamma and specific polyclonal antibody on the infection of murine peritoneal macrophages and murine macrophage cell line PMJ2-R with Encephalitozoon cuniculi. Folia Parasitol. 54:172-176. [PubMed] [Google Scholar]
- 10.Khan, I. A., and M. Moretto. 1999. Role of gamma interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect. Immun. 67:1887-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan, I. A., M. Moretto, and L. M. Weiss. 2001. Immune response to Encephalitozoon cuniculi infection. Microbes Infect. 3:401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodjikian, L., J. G. Garweg, M. Nguyen, T. Schaffner, P. Deplazes, and S. Zimmerli. 2005. Intraocular microsporidiosis due to Encephalitozoon cuniculi in a patient with idiopathic CD4+ T lymphocytopenia. Int. J. Med. Microbiol. 294:529-533. [DOI] [PubMed] [Google Scholar]
- 13.Kolls, J. K., S. Habetz, M. K. Shean, C. Vazquez, J. A. Brown, D. Lei, P. Schwarzenberger, P. Ye, S. Nelson, W. R. Summer, and J. E. Shellito. 1999. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J. Immunol. 162:2890-2894. [PubMed] [Google Scholar]
- 14.Koudela, B., J. Lom, J. Vitovec, Z. Kucerova, O. Ditrich, and J. Travnicek. 1994. In vivo efficacy of albendazole against Encephalitozoon cuniculi in SCID mice. J. Eukaryot. Microbiol. 41:49-50. [PubMed] [Google Scholar]
- 15.Leav, B. A., M. Yoshida, K. Rogers, S. Cohen, N. Godiwala, R. S. Blumberg, and H. Ward. 2005. An early intestinal mucosal source of gamma interferon is associated with resistance to and control of Cryptosporidium parvum infection in mice. Infect. Immun. 73:8425-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina, J. M., M. Tourneur, C. Sarfati, S. Chevret, A. de Gouvello, J. G. Gobert, S. Balkan, and F. Derouin. 2002. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 346:1963-1969. [DOI] [PubMed] [Google Scholar]
- 17.Orenstein, J. M., H. P. Gaetz, A. T. Yachnis, S. S. Frankel, R. B. Mertens, and E. S. Didier. 1997. Disseminated microsporidiosis in AIDS: are any organs spared? AIDS 11:385-386. [PubMed] [Google Scholar]
- 18.Reljic, R. 2007. IFN-gamma therapy of tuberculosis and related infections. J. Interferon Cytokine Res. 27:353-364. [DOI] [PubMed] [Google Scholar]
- 19.Sak, B., J. Salat, H. Horka, K. Sakova, and O. Ditrich. 2006. Antibodies enhance the protective effect of CD4+ T lymphocytes in SCID mice perorally infected with Encephalitozoon cuniculi. Parasite Immunol. 28:95-99. [DOI] [PubMed] [Google Scholar]
- 20.Salat, J., B. Sak, T. Le, and J. Kopecky. 2004. Susceptibility of IFN-gamma or IL-12 knockout and SCID mice to infection with two microsporidian species, Encephalitozoon cuniculi and E. intestinalis. Folia Parasitol. 51:275-282. [PubMed] [Google Scholar]
- 21.Salat, J., H. Horka, B. Sak, and J. Kopecky. 2006. Pure CD4+ T lymphocytes fail to protect perorally infected SCID mice from lethal microsporidiosis caused by Encephalitozoon cuniculi. Parasite Res. 99:682-686. [DOI] [PubMed] [Google Scholar]
- 22.Southern, T. R., C. E. Jolly, M. E. Lester, and. R. Hayman. 2006. Identification of a microsporidia protein potentially involved in spore adherence to host cells. J. Eukaryot. Microbiol. 53:S68-S69. [DOI] [PubMed] [Google Scholar]
- 23.Tremoulet, A. H., M. L. Avila-Aguero, M. M. Paris, A. Canas-Coto, R. Ulloa-Gutierrez, and I. Faingezicht. 2004. Albendazole therapy for microsporidium diarrhea in immunocompetent Costa Rican children. Pediatr. Infect. Dis. 23:915-918. [DOI] [PubMed] [Google Scholar]
- 24.Wang, X., H. Kang, T. Kikuchi, and Y. Suzuki. 2004. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect. Immun. 72:4432-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiwanitkit, V. 2006. Intestinal parasite infestation in HIV-infected patients. Curr. HIV Res. 4:87-96. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, H., H. Huang, A. Cali, P. M. Takvorian, X. C. Feng, G. Zhou, and L. M. Weiss. 2005. Investigations into microsporidian methionine aminopeptidase type 2: a therapeutic target for microsporidiosis. Folia Parasitol. 52:182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]