Abstract
The ability of daptomycin to produce bactericidal activity against Staphylococcus aureus while causing negligible cell lysis has been demonstrated using electron microscopy and the membrane integrity probes calcein and ToPro3. The formation of aberrant septa on the cell wall, suggestive of impairment of the cell division machinery, was also observed.
Many antibiotics derive bactericidal activity from their ability to lyse cells, which may cause liberation of potent proinflammatory bacterial components, resulting in the generation of a robust innate immune response that can potentially cause harm to the host (8). The lipopeptide antibiotic daptomycin is active against a wide range of gram-positive bacteria (Cubicin prescription information, 2005; Cubist Pharmaceuticals, Lexington, MA) (4) and is believed to possess a novel mechanism of action that does not involve cell lysis. Instead, the lipophilic acyl tail of daptomycin is inserted into the cytoplasmic membrane of the bacterium, leading to potassium efflux; destruction of the ion-concentration gradient; membrane depolarization; inhibition of protein, DNA, and RNA synthesis; and finally cell death (Cubicin prescription information, 2005; Cubist Pharmaceuticals, Lexington, MA) (5, 11, 13). Daptomycin is rapidly bactericidal in vitro against Staphylococcus aureus at low multiples of the MIC (12). Here, we demonstrate the bactericidal activity of daptomycin against S. aureus in the absence of cell lysis. (Portions of this work were presented previously at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy [10].)
Cell lysis was initially monitored by measuring optical density during log-phase time kills. Late-exponential-phase cultures (approximately 108 CFU/ml) were used to allow samples to be obtained for transmission electron microscopy (TEM). Staphylococcus aureus ATCC 29213 was grown overnight in calcium-supplemented Mueller-Hinton broth (MHBc; 50 mg/liter Ca2+) and subcultured 1:1,000 into fresh MHBc. Cultures were grown at 37°C with shaking (200 rpm) to an optical density at 600 nm (OD600) of 0.3 to ensure sufficient biomass for fixation and processing. Daptomycin was added at multiples (1× to 8×) of the MIC (0.5 μg/ml). At the indicated time points, samples were removed, OD600 and number of CFU/ml were measured as previously described (7), and cells were pelleted and resuspended in 1 ml MHBc plus 2.5% (vol/vol) glutaraldehyde. Glutaraldehyde-fixed samples were postfixed in 2.0% (wt/vol) osmium tetroxide, followed by en bloc staining with 2.0% (wt/vol) uranyl acetate. The cells were then dehydrated through an ethanol series and embedded in LR White resin. Samples were thin sectioned and stained by uranyl acetate; lead citrate TEM was performed using a LEO 912AB microscope under standard operating conditions at 100 kV, with a liquid nitrogen anticontaminator in place.
As shown in Fig. 1, at 4 μg/ml, daptomycin was rapidly bactericidal, producing a >1,000-fold decrease in viability in less than 120 min, with no concomitant drop in OD600. (Daptomycin displays a well-described inoculum effect [1]; 4 μg/ml is approximately twice the concentration needed to arrest growth at this cell density.) In contrast, OD600 decreased by approximately 50% in cultures treated with the pore-forming antibiotic nisin at a 1× MIC, while viability decreased approximately 200-fold.
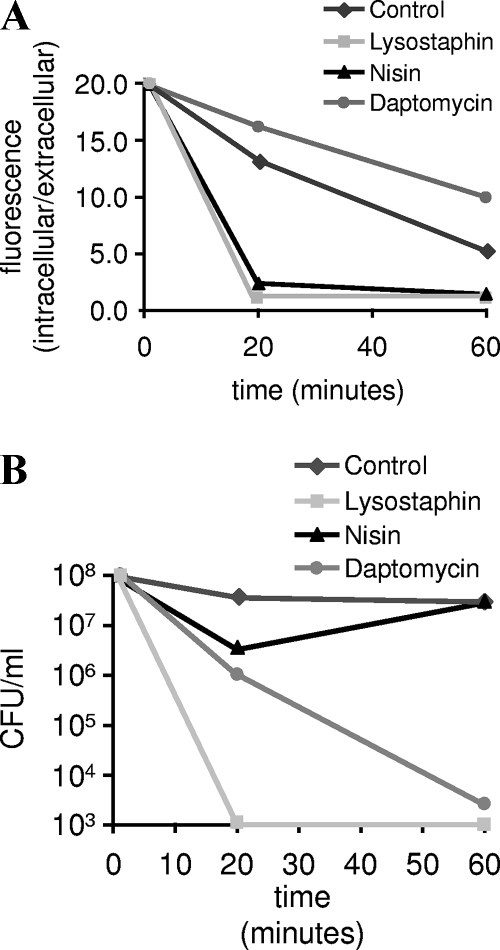
FIG. 1.
Culture density (OD600) and viability were monitored during daptomycin treatment to allow correlation of bactericidal activity and lysis. At 4 μg/ml, daptomycin exhibited significant bactericidal activity with no change in OD600.
Lack of lysis was confirmed by TEM (Fig. 2). There is little evidence of lysis visible in the population of cells treated for 60 min at 4 μg/ml. These results are consistent with those previously reported using scanning electron microscopy (13). Interestingly, >90% of cells display altered cell wall morphology consistent with aberrant division septa.
FIG. 2.
TEM of S. aureus treated with daptomycin (4 μg/ml; 60 minutes) (A) or the control, demonstrating lack of lysis (B). Aberrant division septa and multilobate morphology are visible in >90% of daptomycin-treated cells in this image.
In addition to the assays described above, the integrity of the S. aureus plasma membrane was examined using two fluorometric methods: calcein release and ToPro3 uptake. For the calcein-release assay (6), cells are loaded with the membrane-permeant fluorescent dye calcein-AM. Following uptake, calcein-AM is cleaved to form the membrane-impermeant dye calcein. Release of calcein is monitored fluorometrically following exposure to antibiotics and serves as a marker for membrane damage and cell lysis. As shown in Fig. 3, daptomycin treatment produces rapid bactericidal activity without significant calcein release, consistent with a lack of lysis or even significant structural damage to the cytoplasmic membrane. This is in contrast to lysostaphin, which is bactericidal through the destruction of the S. aureus cell wall and leads to a similar drop in recoverable CFU, but with much greater calcein leakage. The bactericidal activity of the pore-forming antibiotic nisin is also accompanied by significant calcein release. Similar results were obtained using ToPro3, a membrane-impermeant dye whose fluorescence increases significantly in the presence of DNA; increased fluorescence is considered to be a sign of membrane permeability. ToPro3 levels were measured by flow cytometry (Fig. 4) (9). Fluorescence levels were similar in control and daptomycin-treated cells, despite a 1,000-fold loss of viability (not shown). Cells treated with another nonlytic antibiotic (ciprofloxacin) also displayed control-like values, in sharp contrast to those treated with the pore-forming agent nisin. Interestingly, treatment with both the proton ionophore CCCP and the calcium ionophore A23187 actually reduced fluorescence relative to that of untreated controls, suggesting that these agents either decrease normal levels of membrane permeability or interfere with some level of active transport (import or efflux) of ToPro3.
FIG. 3.
(A) Calcein release was monitored following treatment of S. aureus with daptomycin (2 μg/ml), nisin (12.5 μg/ml), and lysostaphin (100 μg/ml). Extracellular dye was separated from cells by passage through a 0.2 μM filter. Data are expressed as ratios of intracellular to extracellular fluorescence. (B) Viability was measured by plating dilute samples (to eliminate antibiotic carryover) on tryptic soy agar.
FIG. 4.
ToPro3 assay showing very similar permeability profiles for daptomycin and the control. Uptake of ToPro3 was monitored by fluorescence-activated cell sorting following treatment with daptomycin (5 μg/ml), ciprofloxacin (2 μg/ml), nisin (25 μg/ml), CCCP (10 μM), and A23187 (1 μg/ml).
The bactericidal ability of daptomycin without cell lysis may have beneficial clinical consequences through the reduced release of proinflammatory bacterial components. For example, daptomycin treatment of methicillin-resistant S. aureus-infected macrophages leads to a 50% reduction in the production of proinflammatory cytokines, such as tumor necrosis factor alpha, compared with vancomycin treatment (2). Similarly, the potential for a lessened inflammatory response compared to that for ceftriaxone has recently been observed in an experimental model of pneumococcal meningitis (3). Further clinical studies are needed to confirm these findings and determine the effect that a lessened inflammatory response might have in humans.
Acknowledgments
This article is dedicated to the memory of the late Terry J. Beveridge, scholar and colleague.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Benson, C. A., F. Beaudette, and G. Trenholm. 1987. Comparitive in vitro activity of LY146032 a new peptolide, with vancomycin and eight other agents against Gram-positive organisms. J. Antimicrob. Chemother. 20:191-196. [DOI] [PubMed] [Google Scholar]
- 2.English, B. K., E. M. Maryniw, A. J. Talatin, and E. M. Meals. 2005. Reduced macrophage tumor necrosis factor (TNF) secretion in response to Staphylococcus aureus (SA) isolates exposed to daptomycin compared with vancomycin or oxacillin. Abstr. Pediatric Academic Societies' Annual Meeting, Washington, DC. abstr. 507.
- 3.Grandgirard, D., C. Schurch, P. Cottagnoud, and S. L. Leib. 2007. Prevention of brain injury by the nonbacteriolytic antibiotic daptomycin in experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 51:2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King, A., and I. Phillips. 2001. The in vitro activity of daptomycin against 514 Gram-positive aerobic clinical isolates. J. Antimicrob. Chemother. 48:219-223. [DOI] [PubMed] [Google Scholar]
- 5.Lakey, J. H., and M. Ptak. 1988. Fluorescence indicates a calcium-dependent interaction between the lipopeptide antibiotic LY146032 and phospholipid membranes. Biochemistry 27:4639-4645. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenfels, R., W. E. Biddison, H. Schulz, A. V. Vogt, and R. Martin. 1994. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J. Immunol. Methods 172:227-239. [DOI] [PubMed] [Google Scholar]
- 7.Mascio, C. T. M., J. D. Alder, and J. A. Silverman. 2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:4255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nau, R., and H. Eiffert. 2005. Minimizing the release of proinflammatory and toxic bacterial products within the host: a promising approach to improve outcome in life-threatening infections. FEMS Immunol. Med. Microbiol. 44:1-16. [DOI] [PubMed] [Google Scholar]
- 9.Novo, D. J., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 2000. Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob. Agents Chemother. 44:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman, J., B. Harris, N. Cotroneo, and T. Beveridge. 2003. Daptomycin (DAP) treatment induces membrane and cell wall alterations in Staphylococcus aureus, abstr. CI-2155. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL.
- 11.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne G. M., and J. Alder. 2002. Daptomycin: a novel lipopeptide antibiotic. Clin. Microbiol. Newsl. 24:33-40. [Google Scholar]
- 13.Wale, L. J., A. P. Shelton, and D. Greenwood. 1989. Scanning electron microscopy of Staphylococcus aureus and Enterococcus faecalis exposed to daptomycin. J. Med. Microbiol. 30:45-49. [DOI] [PubMed] [Google Scholar]