Abstract
We evaluated the in vivo efficacy of three β-cyclodextrin derivatives that block the anthrax protective antigen pore. These compounds were at least 15-fold more potent than previously described β-cyclodextrins in protecting against anthrax lethal toxin in a rat model. One of the drugs was shown to protect mice from bacterial infection.
Bacillus anthracis edema toxin and lethal toxin (LT) are both important virulence factors of anthrax disease which have been shown to replicate many hallmarks of the disease in animal models (4, 10). Protective antigen (PA) is the receptor-binding component common to both of these toxins which forms heptameric transmembrane pores and delivers their respective enzymatic components, edema factor (EF) or lethal factor (LF), to the cell cytosol (16). EF is a calmodulin-dependent adenylate cyclase, and LF is a metalloprotease that cleaves members of the mitogen-activated protein kinase kinase family (9). Inbred mice display a range of sensitivities to LT (9, 11), while the Fischer F344 rat is uniquely sensitive to LT and can succumb as early as 38 min after toxin administration (3) through unknown mechanisms.
The primary target in our arsenal against anthrax disease is PA. Vaccination against this antigen is sufficient to completely protect against anthrax disease in numerous animal models (5, 13, 14). Other than the PA-based vaccine, the only approved therapy for anthrax is the administration of antibiotics after exposure. Antibiotic administration, however, is ineffective as a therapy against anthrax if it is provided after bacterial exposure has led to the production of levels of toxins and other virulence factors sufficient to kill the host. Therefore, the development of direct antitoxin therapeutics which can be provided after exposure to the bacterium is crucial for the treatment of this disease. While a few small-molecule inhibitors of LF have been tested in animal models (15), most studies on PA inhibition focus on the use of monoclonal antibody-mediated therapy (1, 2, 12). Recently, we described one of the only small-molecule inhibitors of PA function and report here on the in vivo efficacy of derivatives of this compound.
We constructed a modified β-cyclodextrin with added positively charged groups which effectively blocked PA channel conductance in vitro, protected against LT-mediated macrophage killing, and was able to rescue LT-treated Fischer F344 rats from death (6). Subsequently, improved per-substituted β-cyclodextrin derivatives with greatly enhanced abilities to block ion conductance through PA channels and to protect against LT toxicity in macrophages at submicromolar concentrations were synthesized and characterized (7). Presented here are the efficacy results of the most promising compounds in two animal models.
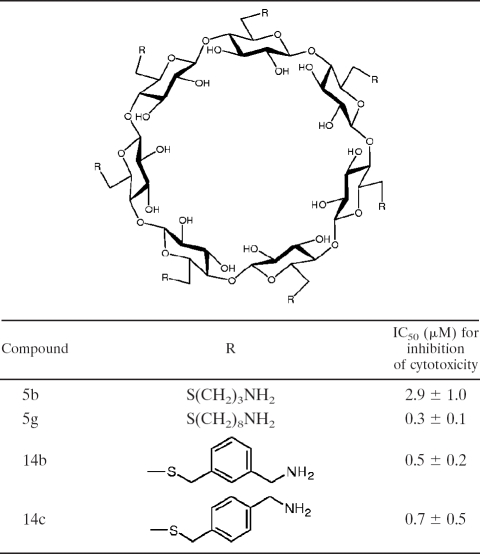
We selected three compounds (compounds 5g, 14b, and 14c) for comparison to our previously tested (6) initial β-cyclodextrin derivative, compound 5b (Table 1), and tested them for their abilities to protect against toxicity when they were coadministered with LT, as well as when they were preadministered. Female Fischer F344 rats (weight, 160 to 185 g; Taconic Laboratories, Germantown, NY) were injected intravenously (i.v.) with LT (10 μg PA plus 10 μg LF) mixed with set amounts of each compound or with phosphate-buffered saline (PBS), and survival was monitored continuously over 8 h. Table 2 summarizes the results from all of the experiments performed. While our initial compound (compound 5b) required a dose of 1.25 mg/rat for protection against LT-induced mortality, a dose of just 0.017 mg/rat of compounds 5g, 14b, and 14c protected two of three animals, and a dose of 0.085 mg/rat protected all six toxin-challenged animals. This represented an almost 15-fold lower dosage for full protection and a greater than 70-fold lower dosage for partial protection. Additionally, pretreatment of the rats (1.25 mg/180 g rat, or 6.75 mg/kg of body weight) with each compound (prepared in PBS, administered i.v.) 30 min prior to toxin challenge was also fully protective (Table 3).
TABLE 1.
β-Cyclodextrin derivatives tested in this study
TABLE 2.
Rat survival with compound cotreatmenta
| Compound | Dose (mg)/rat | Survival (no. of mice that survived/total no.) | Time to death (min) | P valueb |
|---|---|---|---|---|
| PBS | NAc | 0/20 | 72-100 | |
| 5b | 1.25 | 6/6 | <0.0001 | |
| 5b | 0.25 | 0/5 | 130-239 | |
| 5g | 0.25 | 6/6 | <0.0001 | |
| 5g | 0.085 | 6/6 | <0.0001 | |
| 5g | 0.017 | 2/3 | 623 | 0.01186 |
| 14b | 1.25 | 4/4 | <0.0001 | |
| 14b | 0.25 | 7/7 | <0.0001 | |
| 14b | 0.085 | 6/6 | <0.0001 | |
| 14b | 0.017 | 2/3 | 285 | 0.01186 |
| 14c | 0.25 | 6/6 | <0.0001 | |
| 14c | 0.085 | 6/6 | <0.0001 | |
| 14c | 0.017 | 2/3 | 382 | 0.01186 |
Fischer rats were injected i.v. with LT (10 μg PA plus 10 μg LF) mixed with the listed compounds or PBS, and survival was monitored over 8 h.
P values were calculated on the basis of the results for each group relative to those for the PBS-treated controls by the use of Fisher's test (two tailed).
NA, not applicable.
TABLE 3.
Rat survival with compound pretreatmenta
| Compound | Dose (mg)/rat | Survival (no. of mice that survived/total no.) | Time to death (min) | P valueb |
|---|---|---|---|---|
| PBS | NAc | 0/20 | 72-100 | |
| 5b | 1.25 | 3/3 | 0.00056 | |
| 5g | 1.25 | 3/3 | 0.00056 | |
| 14b | 1.25 | 3/3 | 0.00056 | |
| 14c | 1.25 | 3/3 | 0.00056 |
Fischer rats were injected i.v. with each compound 30 min prior to administration of LT (10 μg PA plus 10 μg LF), and survival was monitored over 8 h.
P values were calculated on the basis of the results for each group relative to those for the PBS-treated controls by the use of Fisher's test (two tailed).
NA, not applicable.
One compound (compound 14b) was selected and tested in an infection model of anthrax. Antibiotics alone cannot protect against B. anthracis infection when they are given after sufficient toxin production has occurred. Our mouse model mimics such postsymptomatic B. anthracis infections, and we have successfully used it in our tests of the therapeutic value of polyclonal anti-PA antibody therapy in combination with ciprofloxacin (8). Five groups of 10 DBA/2 mice (age, 9 weeks; average weight, 20 g; Jackson Laboratories, Bar Harbor, ME) were inoculated intraperitoneally (i.p.) with 200 μl of a spore suspension containing 1 × 107 to 1.5 × 107 B. anthracis 34F2 Sterne strain spores (Colorado Serum Company, Denver). At day 1 postchallenge, one group received compound 14b (2.5 mg/kg) alone, a second group received compound 14b at this dose as well as the antibiotic ciprofloxacin (50 mg/kg; MP Biomedicals, Solon, OH), and the last group received only the antibiotic. Compound 14 b was injected i.v., while the antibiotic was administered i.p. Compound 14b and ciprofloxacin were administered in this manner once daily for 10 days postinfection, and the mice were monitored daily for survival. In the representative experiment whose results are shown in Fig. 1, a combination of ciprofloxacin with compound 14b was significantly (P = 0.02) more effective at protecting the mice against B. anthracis infection than antibiotic treatment alone. The results obtained with the less effective ciprofloxacin treatment clearly indicate the problems with late antibiotic therapy in the absence of antitoxin therapeutics once bacterial division has ensued. Repetitions of this experiment provided a rate of survival of up to 90% among animals treated with compound 14b and ciprofloxacin (data not shown). Because compounds like compound 14b described here are among the few small-molecule inhibitors of PA function and have impressive in vivo efficacy, we believe that these β-cyclodextrin derivatives are exciting candidates that merit development as adjunct drugs for the treatment of B. anthracis infection.
FIG. 1.
Protection of mice from B. anthracis infection. Mice challenged with B. anthracis Sterne spores were treated on days 1 to 10 with compound 14b (2.5 mg/kg/day i.v.) alone, with ciprofloxacin (CIPRO) alone (50 mg/kg/day i.p.), or with both compound 14b and ciprofloxacin (by the same schedule used for each compound alone) and survival was monitored. The graph presents the results of a single representative experiment selected to show the lowest survival rates. The combination of ciprofloxacin with compound 14b was significantly (P = 0.02) more effective at protecting the mice than antibiotic treatment alone. In a second set of experiments, 90% of animals treated with compound 14b and ciprofloxacin survived.
Acknowledgments
This research was supported by grant 2R44AI052894-02 from the NIH and by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
We thank Rasem Fattah for producing toxin and Jason Wiggins and Devorah Crown for help with animal experiments and statistical analyses.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Albrecht, M. T., H. Li, E. D. Williamson, C. S. Lebutt, H. C. Flick-Smith, C. P. Quinn, H. Westra, D. Galloway, A. Mateczun, S. Goldman, H. Groen, and L. W. Baillie. 2007. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect. Immun. 75:5425-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, Z., M. Moayeri, Y. H. Zhou, S. Leppla, S. Emerson, A. Sebrell, F. Yu, J. Svitel, P. Schuck, C. M. St. Claire, and R. Purcell. 2006. Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen. J. Infect. Dis. 193:625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezzell, J. W., B. E. Ivins, and S. H. Leppla. 1984. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect. Immun. 45:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firoved, A. M., G. F. Miller, M. Moayeri, R. Kakkar, Y. Shen, J. F. Wiggins, E. M. McNally, W. J. Tang, and S. H. Leppla. 2005. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivins, B., P. Fellows, L. Pitt, J. Estep, J. Farchaus, A. Friedlander, and P. Gibbs. 1995. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine 13:1779-1784. [DOI] [PubMed] [Google Scholar]
- 6.Karginov, V. A., E. M. Nestorovich, M. Moayeri, S. H. Leppla, and S. M. Bezrukov. 2005. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc. Natl. Acad. Sci. USA 102:15075-15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karginov, V. A., E. M. Nestorovich, A. Yohannes, T. M. Robinson, N. E. Fahmi, F. Schmidtmann, S. M. Hecht, and S. M. Bezrukov. 2006. Search for cyclodextrin-based inhibitors of anthrax toxins: synthesis, structural features, and relative activities. Antimicrob. Agents Chemother. 50:3740-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karginov, V. A., T. M. Robinson, J. Riemenschneider, B. Golding, M. Kennedy, J. Shiloach, and K. Alibek. 2004. Treatment of anthrax infection with combination of ciprofloxacin and antibodies to protective antigen of Bacillus anthracis. FEMS Immunol. Med. Microbiol. 40:71-74. [DOI] [PubMed] [Google Scholar]
- 9.Leppla, S. H. 2006. Bacillus anthracis toxins, p. 323-347. In J. E. Alouf and M. R. Popoff (ed.), The comprehensive sourcebook of bacterial protein toxins. Academic Press, Burlington, MA.
- 10.Moayeri, M., D. Haines, H. A. Young, and S. H. Leppla. 2003. Bacillus anthracis lethal toxin induces TNF-α-independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 112:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moayeri, M., N. W. Martinez, J. Wiggins, H. A. Young, and S. H. Leppla. 2004. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 72:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson, J. W., J. E. Comer, D. M. Noffsinger, A. Wenglikowski, K. G. Walberg, B. M. Chatuev, A. K. Chopra, L. R. Stanberry, A. S. Kang, W. W. Scholz, and J. Sircar. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 14.Williamson, E. D., I. Hodgson, N. J. Walker, A. W. Topping, M. G. Duchars, J. M. Mott, J. Estep, C. Lebutt, H. C. Flick-Smith, H. E. Jones, H. Li, and C. P. Quinn. 2005. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect. Immun. 73:5978-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong, Y., J. Wiltsie, A. Woods, J. Guo, J. V. Pivnichny, W. Tang, A. Bansal, R. T. Cummings, B. R. Cunningham, A. M. Friedlander, C. M. Douglas, S. P. Salowe, D. M. Zaller, E. M. Scolnick, D. M. Schmatz, K. Bartizal, J. D. Hermes, M. Maccoss, and K. T. Chapman. 2005. The discovery of a potent and selective lethal factor inhibitor for adjunct therapy of anthrax infection. Bioorg. Med. Chem. Lett. 16:964-968. [DOI] [PubMed] [Google Scholar]
- 16.Young, J. A., and R. J. Collier. 2007. Anthrax toxin: receptor-binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243-265. [DOI] [PubMed] [Google Scholar]