Abstract
Quorum sensing is a mechanism through which a bacterial population receives input from neighboring cells and elicits an appropriate response to enable survival within the host. Inhibiting quorum sensing by RNAIII-inhibiting peptide (RIP) has been demonstrated as a very effective mode of prevention and therapy for device-associated staphylococcal infections and was tested here for healing of wounds that are otherwise resistant to conventional antibiotics. Wounds, established through the panniculus carnosus of BALB/c mice, were inoculated with 5 × 107 CFU of methicillin-resistant Staphylococcus aureus. Mice were treated with Allevyn, RIP-soaked Allevyn (containing 20 μg RIP), daily intraperitoneal teicoplanin (7 mg/kg of body weight), Allevyn and teicoplanin, and RIP-soaked Allevyn and daily intraperitoneal teicoplanin. The main outcome measures were quantitative bacterial culture and histological examination with assessment of microvessel density and of vascular endothelial growth factor (VEGF) expression in tissue sections. Treatment with RIP-soaked Allevyn together with teicoplanin injection greatly reduced the bacterial load to 13 CFU/g (control untreated animals had 108 CFU/g bacteria). All other treatments were also significantly effective but only reduced the bacterial load to about 103 CFU/ml. Histological examination indicated that only treatment with RIP-soaked Allevyn with teicoplanin injection restored epithelial, granulation, and collagen scores, as well as microvessel density and VEGF expression, to the levels found with uninfected mice. In conclusion, we observed that RIP may be useful for the management of infected wounds and that it could represent an exciting and future alternative to the conventional antibiotics, at present considered the gold-standard treatments for methicillin-resistant S. aureus infections.
The individual patient with a chronic wound is not much better served today than a decade ago, despite all the advances in our understanding. For example, the outcomes for a patient with a diabetic foot ulcer remain bleak. The rate of major limb amputations is not decreasing, the mortality associated with major limb amputations is not declining, and the quality of life reported by these patients is not improving (9, 29). Many of the nonhealing complex wounds show a Staphylococcus species in culture, and many of these wounds do not respond to commercially available agents such as appropriate antibiotics, selective biocides, advanced dressings, etc.
There is evidence that chronic wounds are different from acute wounds because they possess biofilm on their surface (17). Biofilms are structured communities of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface. It is estimated that about 60% of all microbial infections involve bacterial biofilms (13, 23, 26). The biofilm mode of growth enables the bacteria to survive exposure to various antibiotics at concentrations 1,000-fold higher than their growing counterparts (32). Relative resistance can be due to restricted penetration of antimicrobials or heterogeneous metabolic activity, as antimicrobials mostly target metabolically active cells, so slow or nongrowing parts of the biofilms are very difficult to target. Some antibiotics have reduced activity in oxygen-deprived environments, which also contributes to biofilm resistance, as availability of oxygen is reduced in the deeper levels of a biofilm (16, 23). Another option is the expression of certain genes in a biofilm, conferring enhanced resistance to antibiotics (30, 34). In staphylococci, for example, persistence within a biofilm requires an adaptive response that limits the deleterious effects of the reduced pH associated with anaerobic growth conditions (6), as well as the production of multiple virulence factors that allow the bacteria to survive within the host (24). These genes have been shown to be regulated by cell-to-cell communication (quorum sensing) (20, 25).
The underlying mechanism of quorum sensing is the production of diffusible chemical signal molecules by the bacteria (autoinducers) that interact with specific receptors on themselves and on neighboring cells, which in turn regulate expression of specific target genes. By integrating this with other environmental signals and stimuli, bacteria are capable of exhibiting complex responses and take part in sophisticated interactions (25). In staphylococci, quorum sensing regulates both biofilm formation and toxin production, and it can be inhibited by RNAIII-inhibiting peptide (RIP) (18). RIP is a heptapeptide (YSPWTNF-NH2), and in its presence, the bacteria are no longer virulent and do not cause disease. RIP has been shown to be extremely effective in preventing and treating drug-resistant device-associated staphylococcal infections, including those caused by methicillin-resistant and vancomycin-intermediate S. aureus and S. epidermidis strains (2, 3, 5, 10, 11).
It is well known that wound healing is regulated by a pattern of events including coagulation, inflammation, formation of granulation tissue, epithelization, and tissue remodeling (31). All events are mediated and modulated by interacting molecular signals, primarily cytokines and growth factors that stimulate the main cellular activities, which underscore the healing process (14, 31). That, combined with increasing resistance of staphylococci to antibiotics (24), led us to test the efficacy of RIP and a commonly used dressing, Allevyn (15), on the treatment of experimental surgical wound infections caused by methicillin-resistant S. aureus (MRSA) and to test the host cell response to treatment.
MATERIALS AND METHODS
Organisms.
The commercially available MRSA strain ATCC 43300 was used.
Animals.
Adult male BALB/c mice weighing 35 to 50 g were used for all the experiments (n = 12 per group). All animals were housed in individual cages under constant temperature (22°C) and humidity with a 12-h light/dark cycle, and they had access to chow and water as much as desired throughout the study. The environment was temperature and humidity controlled, with lights on and off at 6:30 a.m. and 6:30 p.m. The study was approved by the animal research ethics committee of the I.N.R.C.A.-I.R.R.C.S., Università Politecnica delle Marche, Ancona, Italy.
Drugs.
The amide form of RIP (YSPWTNF-NH2) was synthesized by Neosystem (Strasbourg, France) and purified by high-pressure liquid chromatography (HPLC) to 99%. RIP powder was dissolved in distilled H2O at 20 times the required maximal concentration. Teicoplanin (Aventis Pharma S.p.A., Rome, Italy) was diluted in accordance with the manufacturer's recommendations, yielding a 10-mg/ml stock solution. Solutions were made fresh on the day of assay or stored at −80°C in the dark for short periods. The concentration range assayed for the MIC of teicoplanin was 0.25 to 256 mg/liter.
MIC determination.
The MIC was determined according to the procedures outlined by the Clinical and Laboratory Standards Institute (CLSI) (12). It was taken as the lowest drug concentration at which observable growth was inhibited. Experiments were performed in triplicate.
Absorbance and elution profiles of RIP by Allevyn.
Pieces (1 by 2 cm2) of Allevyn (Smith & Nephew) were placed in 20 ml RIP (10 μg/ml). After 20 min, Allevyn was removed and the remaining solution was saved (unbound). Allevyn pieces were placed in 5 ml water to determine the elution profile, where 1 ml solution was removed after 2, 5, and 24 h. One-milliliter unabsorbed and eluted fractions were applied to a HPLC reverse-phase column (150 by 4.6 mm; Hypersil Gold; Thermo Electron Corp.) and eluted by 0 to 70% acetonitrile in a 0.1% trifluoroacetic gradient at a flow rate of 1 ml/min. Peptide that eluted at about 50% acetonitrile was detected spectroscopically at 216 nm or 280 nm. Of note is that within minutes, 2 ml RIP solution was absorbed by the Allevyn 1- by 2-cm2 pieces.
Preparation of inoculum.
Bacteria were grown in brain-heart infusion broth. When bacteria were in the log phase of growth, the suspension was centrifuged at 1,000 × g for 15 min, the supernatant was discarded, and the bacteria were resuspended and diluted into sterile saline to achieve a concentration of approximately 5 × 107 CFU/ml.
Mouse wound infection model.
The study included a control group that did not receive any treatment, a control group that received RIP local treatment, a contaminated group that did not receive any treatment, and five contaminated groups treated, respectively, with (i) drug-free Allevyn (Smith & Nephew Healthcare, St. Hull East Riding of Yorkshire, United Kingdom), (ii) RIP-soaked Allevyn, (iii) daily intraperitoneal teicoplanin (7 mg/kg of body weight), (iv) drug-free Allevyn and daily intraperitoneal teicoplanin (7 mg/kg), and finally, (v) RIP-soaked Allevyn (containing 20 μg RIP) and daily intraperitoneal injections of teicoplanin (7 mg/kg). The main outcome measures were quantitative culture and histological examination of tissue repair and assessment of microvessel density (MVD) and vascular endothelial growth factor (VEGF) expression in endothelial cells and fibroblasts in excised tissues.
Mice were anesthetized by an intramuscular injection of ketamine (50 mg/kg) and xylazine (8 mg/kg), and hair on the back was shaved and the skin cleansed with 10% povidone-iodine solution. Using a 1.0- by 2.0-cm template, one full-thickness wound was established through the panniculus carnosus on the back subcutaneous tissue of each animal. A small gauze patch was placed over each wound and then inoculated with 5 × 107 CFU of S. aureus ATCC 43300. The pocket was closed by means of skin clips (21). This procedure results in a local abscess at 24 h. One wound was created per animal. The animals were returned to individual cages and thoroughly examined daily. After 24 h, the wounds in control animals were opened and the gauze removed for quantitative bacterial culture, and then treatment was initiated. Intraperitoneal teicoplanin (7 mg/kg) was administered daily for 7 days, while topical treatment (RIP and Allevyn with or without teicoplanin) was applied every 2 days. Peptide absorption was obtained immediately before implantation by soaking Allevyn for 20 min in a sterile RIP solution (10 mg/liter), resulting in 20 μg RIP on the 1- by 2-cm2 Allevyn.
Animals were euthanized and 1- by 2-cm areas of skin, including the wounds, were excised aseptically. Skin samples were divided in two. One piece was used for histological examination (see below), and the other was homogenized in 1 ml phosphate-buffered saline using a stomacher. Quantization of viable bacteria was performed by culturing serial dilutions (0.1 ml) of the bacterial suspension on blood agar plates. All plates were incubated at 37°C for 48 h and evaluated for the presence of bacteria. The organisms were quantitated by counting the number of CFU per plate. The limit of detection for this method was approximately 10 CFU/g.
Histological examination of excised tissues and assessment of angiogenesis.
Surgically removed samples were collected at the time of euthanasia and were fixed in 10% buffered formalin, dehydrated in alcohol, cleared in xylene, and paraffin embedded (see below). Five-micrometer tissue sections, including the epidermis, the dermis, and the subcutaneous panniculus carnosus muscle, were cut and stained with hematoxylin and eosin and Masson's trichrome. All subsequent analyses were performed by an observer blinded to the treatment. The specimens were assessed under light microscopy for the progression of new epithelium, inflammation, vascular responses, and the formation of collagen in the wound defect. Morphological features such as degree of reepithelization, granulation tissue formation, and collagen organization were scored, as well as the presence or absence of bacteria (Table 1) (19).
TABLE 1.
Score of morphological features
| Score | Reepithelization | Granulation tissue formation | Collagen organization |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Migrating | Hypocellular with few vessels | Trace |
| 2 | Partial stratum corneum | Many vessels and some cells | Slight |
| 3 | Hypertrophic | Many fibroblasts, some fibers | Moderate |
| 4 | Complete and normal | More fibers, few cells | Marked |
The angiogenetic process in the infected wounds under different treatment regimens was evaluated by assessing the MVD and the endothelial cell VEGF expression by immunohistochemistry. Five-micrometer paraffin-embedded tissue sections on poly-l-lysine-coated glass slides were heat-dried, deparaffinized in xylene, and sequentially rehydrated in gradients of ethanol. Sections were treated with TUF solution (Histo-line Laboratories) at 90°C for 10 min to better unmask antigenic sites. After three washes with H2O, sections were incubated overnight at 4°C with rabbit anti-VEGF-C-1 (diluted 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-CD31/PECAM-1 (diluted 1:20; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. Antibody binding was localized using a secondary biotin-labeled goat anti-rabbit antibody which was then detected using streptavidin-conjugated horseradish peroxidase (Dako Cytomation, Milano, Italy) as follows: sections were incubated with 3,3′-diaminobenzidine (0.05 diaminobenzidine in 0.05 M Tris buffer, pH 7.6, and 0.01% hydrogen peroxide) and counterstained with Mayer's hematoxylin. The MVD was assessed in selected fields by counting the number of CD31-positive small vessels under a light microscope at a magnification of ×400 covering an area of within 0.16 mm2 per field. Any brown-stained endothelial cell or endothelial cell cluster that was clearly separated from adjacent microvessels was considered a single countable microvessel. MVD was then expressed as the number of counted microvessels per mm2. The endothelial cell reactivity for VEGF was expressed as the percentage of positive cells counted in the selected fields under a light microscope. All counts were performed by one investigator three times for each sample and expressed as the obtained mean values.
Statistical analysis.
For efficacy, the outcome measures for comparison of treatments were the number of bacteria in excised tissues, the histological scoring system for wound repair on sections, and MVD and VEGF expression in fibroblasts and endothelial cells in granulation tissues. All results are presented as group means with standard deviations. Statistical analysis was performed using an analysis of variance test. Significance was accepted when the P value was <0.05.
RESULTS
In the present study, a mouse model of surgical wound infection was used to investigate the efficacy of drug-free and RIP-soaked hydrocellular foam compared to that of parenteral administration of teicoplanin. The main outcome measures in the study were quantitative culture of excised tissues, histological examination, and assessment of MVD and endothelial cell VEGF expression.
Quantitative culture of excised tissues.
Teicoplanin exhibited an MIC of 1 mg/liter. RIP did not demonstrate any in vitro activity against the strain (MIC of >256 mg/liter), as expected by its mechanism of action.
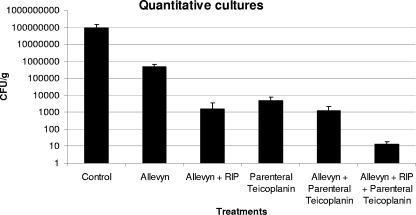
Mean bacterial numbers in challenged but untreated controls (8.8 × 107 ± 1.5 × 107 CFU/ml) were significantly higher than those recovered from all treatment groups (Fig. 1). Specifically, Allevyn alone reduced bacterial numbers to 4.8 × 105 ± 1.0 × 105 CFU/ml. The combination of RIP and Allevyn reduced bacterial load to 1.6 × 103 ± 0.4 × 103 CFU/ml. A reduction in bacterial load was also obtained following administration of intraperitoneal teicoplanin with or without Allevyn (1.2 × 103 ± 0.2 × 103 and 4.8 × 103 ± 0.3 × 103, respectively). Finally, the highest inhibition of bacterial load was obtained in the group that received RIP-soaked Allevyn and intraperitoneal teicoplanin (1.3 × 101 ± 0.1 × 101) (P < 0.01). No abnormal behavioral patterns, such as fatigue, stress, aggressiveness, weight loss, or change in movement, were observed among the mice at any time during the course of these experiments. Finally, no animal displayed signs of deeper infections, such as fever, abscesses, or inflammatory signs.
FIG. 1.
Bacterial loads in wounds of untreated and treated animals.
Histological examination of excised tissues and assessment of angiogenesis.
The scoring system was used to enumerate the recovery status of wounded animals. As shown in Table 2, control wounded but uninfected mice showed an average epithelization score of 2.6, granulation score of 2.9, and collagen organization score of 2.3 (2.6/2.9/2.4) (Fig. 2A). Similar results (average epithelization score of 2.6, granulation score of 2.7, and collagen organization score of 2.2) were observed in control wounded uninfected mice treated with RIP, except for a slight increase of inflammatory cells in the granulation tissue. Infected mice showed average epithelial/granulation/collagen scores of 1/2/0.75, respectively (Fig. 2B). When mice were treated with drug-free Allevyn or parenteral administration of teicoplanin, scores were 1.6/2/1.5 (Fig. 2C and E). When treated with both Allevyn and teicoplanin, scores were 1.75/2.3/1.75 (Fig. 2D). Scores were best for RIP-soaked Allevyn (2.6/2.9/2.4; Fig. 2F) and for RIP-soaked Allevyn and daily parenteral teicoplanin (2.7/2.7/2.7); a slight increase of inflammatory cells was also observed. All treatment groups scored significantly (P < 0.001) better than the control untreated group (Table 2).
TABLE 2.
Summary of the effects of different treatment modalities on various healing parameters at 7 days postwounding in mouse modelsa
| Mouse model | Score (mean ± SD)
|
||
|---|---|---|---|
| Reepithelization | Granulation tissue formation | Collagen organization | |
| Noninfected, nontreated mice | 2.63 ± 0.50 | 2.90 ± 0.94 | 2.36 ± 0.50 |
| Noninfected mice treated with RIP | 2.60 ± 0.42 | 2.75 ± 0.44 | 2.41 ± 0.77 |
| MRSA-infected mice without treatment | 1.08 ± 0.66 | 2.08 ± 0.51 | 0.75 ± 0.38 |
| MRSA-infected mice treated with: | |||
| Drug-free Allevyn | 1.66 ± 0.88 | 2.16 ± 0.71 | 1.41 ± 0.66 |
| Drug-free Allevyn and daily i.p. teicoplanin | 1.75 ± 0.86 | 2.33 ± 0.49 | 1.75 ± 0.86 |
| Daily i.p. teicoplanin | 1.66 ± 0.51 | 2.18 ± 0.40 | 1.67 ± 0.9 |
| RIP-soaked Allevyn | 2.66 ± 0.91* | 2.91 ± 0.53* | 2.41 ± 0.70* |
| RIP-soaked Allevyn and daily i.p. teicoplanin | 2.72 ± 0.46* | 2.78 ± 0.51* | 2.70 ± 0.91* |
The RIP-treated group (with or without i.p. teicoplanin) showed significant improvements of all parameters related to wound healing compared to the values for MRSA-infected mice without treatment and all the other treated groups. *, P < 0.001 (analysis of variance test); i.p., intraperitoneal.
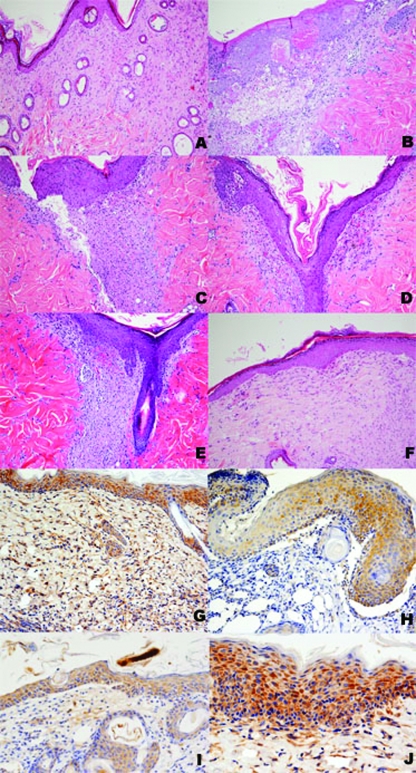
FIG. 2.
Histological features of wounds of untreated and treated animals. (A) A wound from a noninfected mouse is shown with a complete and normal epidermis (reepithelization score of 4), a collagenized granulation tissue with few fibroblasts (granulation tissue formation score of 4), and a marked collagen organization (score of 4). (B) A wound from a MRSA-infected mouse without treatment shows an incomplete epidermis (reepithelization score of 1), a hypocellular granulation tissue with few vessels (granulation tissue formation score of 1), and traces of collagen organization (score of 1). Treatment with drug-free Allevyn (C), drug-free Allevyn and daily parenteral or intraperitoneal teicoplanin (D), and daily parenteral or intraperitoneal teicoplanin alone (E) resulted in a higher degree of wound repair with a hypertrophic epidermis (reepithelization score of 3) in all cases, granulation tissue with many vessels and some fibroblasts and inflammatory cells (granulation tissue formation score of 2), and moderate collagen organization (score of 3). (F) A wound treated with RIP-soaked Allevyn bears many similarities with the case depicted in panel A, with a complete and normal epidermis (reepithelization score of 4), a collagenized granulation tissue with few fibroblasts (granulation tissue formation score of 4), and a marked collagen organization (score of 4). Panels G to J show immunostaining for VEGF in wounds of untreated and treated animals. (G) A wound from a noninfected mouse shows strong immunostaining in epidermal and adnexal epithelial cells as well as in fibroblasts and vessels of the dermis. (H) A wound from a MRSA-infected mouse without treatment shows faint to moderate staining only in the epidermis and not in the dermal fibroblastic, inflammatory, and endothelial cells. Treatment with drug-free Allevyn (I) resulted in a higher degree of VEGF expression in the epidermis and in a moderate number of vessels, fibroblasts, and inflammatory cells. (J) A wound treated with RIP-soaked Allevyn bears many similarities with the case depicted in panel G, with strong immunostaining in epidermal epithelial cells as well as in fibroblasts and vessels of the dermis.
The MVD and VEGF expression were lower (156.53 ± 30.73 and 296.32 ± 38.83, respectively; P < 0.001) in mice infected and not treated than in the noninfected nontreated mice and in all treatment groups (Table 3). Most remarkable was the finding that the group treated with RIP-soaked Allevyn associated with parenteral teicoplanin showed the highest mean values of MVD and VEGF expression compared to the other treatment groups (276.19 ± 40.7 and 473.96 ± 44.17, respectively; P < 0.001) (Table 3). VEGF was expressed by granulation tissue cells, migrating epidermal cells, and hair follicles adjacent to the injured area.
TABLE 3.
Quantitative MVD and number of CD31-positive cells at 7 days postwounding in mouse modelsa
| Mouse model | MVD (no. of small vessels/mm2) | VEGF (no. of positive cells/mm2) |
|---|---|---|
| Noninfected, nontreated mice | 229.64 ± 33.42 | 464.45 ± 36.49 |
| Noninfected mice treated with RIP | 220.88 ± 41.34 | 448.22 ± 34.76 |
| MRSA-infected mice without treatment | 156.53 ± 30.73 | 296.32 ± 38.83 |
| MRSA-infected mice treated with: | ||
| Drug-free Allevyn | 231.63 ± 71.57 | 363.04 ± 126.15 |
| Drug-free Allevyn and daily i.p. teicoplanin | 227.53 ± 33.51 | 374.65 ± 54.87 |
| Daily i.p. teicoplanin | 215.03 ± 16.19 | 373.78 ± 22.04 |
| RIP-soaked Allevyn | 238.49 ± 28.22 | 429.45 ± 19.56 |
| RIP-soaked Allevyn and daily i.p. teicoplanin | 276.19 ± 40.7* | 473.96 ± 44.17* |
The RIP-treated group (with or without i.p. teicoplanin) showed a significant increase of the vascular network compared to that for MRSA-infected mice without treatment. Moreover, only the RIP-treated group with i.p. teicoplanin showed a significant increase of the vascular network compared to those of all the other treated groups. *, P < 0.001 (analysis of variance test); i.p., intraperitoneal.
In the RIP-soaked Allevyn treatment group, the immunoreactivity for VEGF protein as well as the MVD appeared not to be significantly increased compared to those for all the other treatment groups. Uninfected wounds treated with RIP showed MVD and VEGF expression similar to those for untreated uninfected wounds.
Absorbance and elution of RIP by Allevyn.
Allevyn was placed in a 10-μg/ml RIP solution, and absorbance and elution profiles were determined by reverse-phase HPLC. Within minutes, 1- by 2-cm2 Allevyn pieces absorbed 2 ml solution, suggesting that each Allevyn piece placed on the animal contained 20 μg RIP. RIP-containing Allevyn pieces were then placed in water for 2, 5, and 24 h, and fractions were tested by reverse-phase HPLC for the presence of eluted RIP. No detectable amounts of eluted RIP were found in the eluted fractions, suggesting that in the conditions tested, RIP did not elute from Allevyn.
DISCUSSION
In our animal model, full-thickness wounds allowed S. aureus to colonize the skin, and this colonization was associated with an inflammatory host response, as determined by histology. The results of the in vivo study demonstrated that the use of RIP-soaked Allevyn alone or with an antibiotic like teicoplanin was useful in the management of infected wounds because of a significant bacterial inhibition and accelerated repair process.
In S. aureus, multiple quorum-sensing-regulated toxins are produced and have been shown to be important for pathogenesis (5, 6, 18). S. aureus has remained an important cause of nosocomial wound infections, and antibiotic-resistant strains are on the rise (24). A novel way to prevent staphylococcal infections would be to interfere with the quorum sensing that leads to the production of toxins and to the virulent phenotype.
Here we present new insights into the efficacy of the treatment of topical staphylococcal infection. Moreover, in this experimental study, hydrocellular foams were studied to investigate the potential advantage of their inhibition with RIP solution. Clinical experience concerning the tie-over dressing techniques demonstrates that hydrocellular dressings are effective and comfortable in the treatment of various wound types (1, 8). We chose to also evaluate teicoplanin because glycopeptides are considered the gold-standard treatment for staphylococcal infections (28).
Our data indicate that wound remodeling after 1 week was not complete in mice infected and not treated, showing an incomplete epidermal lining in most of the cases, persistence of abundant fibrinous exudation, a small amount of granulation tissue, and rich inflammatory cells with a small amount of vessels and fibroblasts. In all the treated groups, the process of healing was observed to have variable degrees of reduction of fibrinous exudation, enlargement of granulation tissue, moderate deposition of collagen, and reconstitution of the epidermal lining. In particular, infected mice receiving RIP-soaked Allevyn exhibited a higher degree of inflammatory cells in the granulation tissue than did mice receiving drug-free Allevyn and parenteral teicoplanin alone. The group treated with RIP-soaked Allevyn associated with parenteral teicoplanin showed the highest mean values of the parameters related to the wound healing compared to the values for all the other treatment groups, except for the granulation tissue value, which was slightly but significantly reduced with respect to the group treated only with RIP-soaked Allevyn. Results from uninfected wounds treated with topical RIP seem to confirm that RIP itself induces an increase of inflammatory cells in the granulation tissue.
It is well established that wound healing is a complex process and angiogenesis plays a pivotal role in the process. In fact, angiogenesis involves the growth of capillaries from preexisting blood vessels and it is normally found during embryonic development while it is almost absent in the adult. It has been shown that there are some selective mitogenic factors for endothelial cells: after a trauma, these important physiological regulators act to produce an acute local inflammatory response with aggregation of platelets. Several cytokines are released by aggregate platelets after degranulation. Among these molecules, VEGF is a vascular permeability factor that increases extravasation of plasma proteins to create nutritive support for endothelial and epithelial cells. It is an endothelial cell-specific mitogen in vitro and an angiogenic inducer in a variety of in vivo models. Increased plasma levels of VEGF have been found in the presence of a wide range of tumors and during wound healing (7, 15, 33, 35). For these reasons, in the present study the measurement of endothelial cell VEGF expression in excised tissues was considered to be a main outcome measure together with quantitative culture and histological examination. Our data on wound tissues provide strong evidence that treatment with RIP stimulates the process of angiogenesis and up-regulation of VEGF expression throughout wound granulation tissue in infected wounds. These findings might be related to the recruitment of a robust amount of inflammatory cells induced by RIP treatment, where neutrophils and macrophages are the principal components among cells that invade wound areas and constitute the major source of angiogenic growth factors (27). In the other treatment groups (drug-free Allevyn and drug-free Allevyn and daily intraperitoneal teicoplanin), the process of angiogenesis appeared to be relatively reduced with respect to the RIP treatment group, suggesting that persistence of infection is associated with enzymatic activity, which might affect VEGF stability. In fact, bacteria have been shown to contribute to proteolysis in ulcers by facilitating generation of plasmin on their surfaces (22). RIP is known to repress staphylococcal toxin production (4). Moreover, it seems not to have a direct effect on the host because, as we observed in our study, RIP-treated uninfected wounds showed angiogenesis similar to that of the uninfected control group. We believe that further studies addressing mechanisms of normal and pathological cutaneous function are needed to evaluate the possible activity of RIP on wound healing and angiogenesis.
To summarize, wound-related infections can become chronic, leading to economic loss secondary to patient morbidity and possible mortality. Many of these infections involve Staphylococcus spp. which are capable of developing antibiotic resistance. Community-acquired or nosocomial microbial antibiotic resistance is eroding the miracle of antibiotics and jeopardizing both human and animal welfare. With evidence of an increased frequency of resistance to vancomycin, our most-potent current antibiotic, the drive is on to develop novel therapeutic agents. RIP therapy represents an exciting alternative.
Acknowledgments
We express our thanks to Silvana Esposito for her technical assistance.
This work was supported by the Italian Ministry of Education, University and Research (PRIN 2005).
Naomi Balaban is a consultant to the company Centegen Inc., who licensed RIP patents.
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Akiyama, H., H. Kanzaki, Y. Abe, J. Tada, and J. Arata. 1994. Staphylococcus aureus infection on experimental croton oil-inflamed skin in mice. J. Dermatol. Sci. 8:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Anguita-Alonso, P., A. Giacometti, O. Cirioni, R. Ghiselli, F. Orlando, V. Saba, G. Scalise, M. Sevo, M. Tuzova, R. Patel, and N. Balaban. 2007. RNAIII-inhibiting-peptide-loaded polymethylmethacrylate prevents in vivo Staphylococcus aureus biofilm formation. Antimicrob. Agents Chemother. 51:2594-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban, N., A. Giacometti, O. Cirioni, Y. Gov, R. Ghiselli, F. Moccheggiani, C. Viticchi, M. S. Del Prete, V. Saba, G. Scalise, and G. Dell'Acqua. 2003. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 187:625-630. [DOI] [PubMed] [Google Scholar]
- 4.Balaban, N., T. Goldkorn, Y. Gov, M. Hirshberg, N. Koyfman, H. R. Matthews, R. T. Nhan, B. Singh, and O. Uziel. 2001. Regulation of Staphylococcus aureus pathogenesis via target of RNAIII activating protein (TRAP). J. Biol. Chem. 276:2658-2667. [DOI] [PubMed] [Google Scholar]
- 5.Balaban, N., T. Goldkorn, R. T. Nhan, L. B. Dang, S. Scott, R. M. Ridgley, A. Rasooly, S. C. Wright, J. W. Larrick, R. Rasooly, and J. R. Carlson. 1998. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science 280:438-440. [DOI] [PubMed] [Google Scholar]
- 6.Beenken, K. E., P. M. Dunman, and F. McAleese. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjellerup, M. 2005. Novel method for training skin flap surgery: polyurethane foam dressing used as a skin equivalent. Dermatol. Surg. 31:1107-1111. [DOI] [PubMed] [Google Scholar]
- 8.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzato, M. A., E. C. Tribulatto, S. M. Costa, W. G. Zorn, and B. van Bellen. 2002. Major amputations of the lower leg. The patients two years later. Acta Chir. Belg. 102:248-252. [DOI] [PubMed] [Google Scholar]
- 10.Cirioni, O., A. Giacometti, R. Ghiselli, G. Dell'Acqua, F. Orlando, F. Mocchegiani, C. Silvestri, A. Licci, V. Saba, G. Scalise, and N. Balaban. 2006. RNAIII-inhibiting peptide significantly reduces bacterial load and enhances the effect of antibiotics in the treatment of central venous catheter-associated Staphylococcus aureus infections. J. Infect. Dis. 193:180-186. [DOI] [PubMed] [Google Scholar]
- 11.Cirioni, O., A. Giacometti, R. Ghiselli, G. Dell'Acqua, Y. Gov, W. Kamysz, J. Lukasiak, F. Mocchegiani, F. Orlando, G. D'Amato, N. Balaban, V. Saba, and G. Scalise. 2003. Prophylactic efficacy of topical temporin A and RNAIII-inhibiting peptide in a subcutaneous rat pouch model of graft infection due to staphylococci with intermediate resistance to glycopeptides. Circulation 108:767-771. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6. CLSI, Wayne, PA.
- 13.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 14.Crovetti, G., G. Martinelli, M. Issi, M. Barone, M. Guizzardi, B. Campanati, M. Moroni, and A. Carabelli. 2004. Platelet gel for healing cutaneous chronic wounds. Transfus. Apher. Sci. 30:145-151. [DOI] [PubMed] [Google Scholar]
- 15.Dinar, S. 2006. A new material for the standard burn model: Allevyn adhesive. Plast. Reconstr. Surg. 117:717-718. [DOI] [PubMed] [Google Scholar]
- 16.Drenkard, E. 2003. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 5:1213-1219. [DOI] [PubMed] [Google Scholar]
- 17.Enoch, S., and K. G. Harding. 2003. Science behind the removal of barriers to healing. Wounds 15:213-229. [Google Scholar]
- 18.Gov, Y., A. Bitler, G. Dell'Acqua, J. V. Torres, and N. Balaban. 2001. RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus: structure and function analysis. Peptides 22:1609-1620. [DOI] [PubMed] [Google Scholar]
- 19.Hebda, P. A., D. Whaley, H. G. Kim, and A. Wells. 2003. Absence of inhibition of cutaneous wound healing in mice by oral doxycycline. Wound Rep. Reg. 11:373-379. [DOI] [PubMed] [Google Scholar]
- 20.Korem, M., Y. Gov, M. D. Kiran, and N. Balaban. 2005. Transcriptional profiling of target of RNAIII-activating protein, a master regulator of staphylococcal virulence. Infect. Immun. 73:6220-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kugelberg, E., T. Norstrom, T. K. Petersen, T. Duvold, D. I. Andersson, and D. Hughes. 2005. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 49:3435-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauer, G., S. Sollberg, M. Cole, I. Flamme, J. Stürzebecher, K. Mann, T. Krieg, and S. A. Eming. 2000. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J. Investig. Dermatol. 115:12-18. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowy, F. D. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J. Clin. Investig. 111:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.March, J. C., and W. E. Bentley. 2004. Quorum sensing and bacterial cross-talk in biotechnology. Curr. Opin. Biotechnol. 15:495-502. [DOI] [PubMed] [Google Scholar]
- 26.Matz, C., T. Bergfeld, S. A. Rice, and S. Kjelleberg. 2004. Microcolonies, quorum sensing and cytotoxicity determine the survival of Pseudomonas aeruginosa biofilms exposed to protozoan grazing. Environ. Microbiol. 6:218-226. [DOI] [PubMed] [Google Scholar]
- 27.Naldini, A., and F. Carraro. 2005. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm. Allergy 4:3-8. [DOI] [PubMed] [Google Scholar]
- 28.Pace, J. L., and G. Yang. 2006. Glycopeptides: update on an old successful antibiotic class. Biochem. Pharmacol. 71:968-980. [DOI] [PubMed] [Google Scholar]
- 29.Peters, E. J., M. R. Childs, R. P. Wunderlich, L. B. Harkless, D. G. Armstrong, and L. A. Lavery. 2001. Functional status of persons with diabetes-related lower-extremity amputations. Diabetes Care 24:1799-1804. [DOI] [PubMed] [Google Scholar]
- 30.Sauer, K., A. K. Camper, G. H. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinzawa, H., A. Takeda, Y. Sone, K. Murashita, and E. Uchinuma. 2007. Wound healing process of a full-thickness skin wound model in rats. Int. Surg. 92:63-72. [PubMed] [Google Scholar]
- 32.Teitzel, G. M., and M. R. Parsek. 2003. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaingankar, N. V., P. Sylaidis, V. Eagling, C. King, and F. Elender. 2001. Comparison of hydrocellular foam and calcium alginate in the healing and comfort of split-thickness skin-graft donor sites. J. Wound Care 10:289-291. [DOI] [PubMed] [Google Scholar]
- 34.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 35.Williams, C. 1995. Allevyn. Br. J. Nurs. 4:107-108, 110. [DOI] [PubMed] [Google Scholar]