Abstract
We describe a case of recurring Candida glabrata infection in a 68-year-old African-American female on caspofungin therapy. The initial isolate was susceptible, but isolates recovered during following relapses were not. All isolates were clonal, and high-MIC strains contained a mutation in the highly conserved hot spot 1 region of Fks1p.
Risk factors for acquisition of Candida glabrata bloodstream infections are somewhat dissimilar to those for other Candida species (3). A recent case control trial identified 55 C. glabrata cases between 1997 and 2004 in which older age, gastrointestinal disorders, and crude mortality rate (42% versus 29%) were significantly different from those for C. albicans infections. In a prospective (2001-2004) sentinel surveillance study including the previous patients, caspofungin susceptibilities were determined for 8,197 invasive strains of Candida. Almost all Candida spp. had a caspofungin MIC of 0.03 μg/ml at 24 h, with 98.2% of strains being inhibited at concentrations of ≤0.5 μg/ml and 99.7% being inhibited at concentrations of ≤1 μg/ml. Only one C. glabrata isolate showed a MIC of >1.0 μg/ml. During these early years of echinocandin use, no significant changes in invasive Candida susceptibilities were observed (16).
Echinocandins reportedly provide clinicians with a relatively broad anticandidal option with limited toxicity in patients (5). However, cases of echinocandin resistance, typically involving oropharyngeal disease with long-term drug exposure, have recently been observed. More disconcerting was the fact that we observed a case of reduced C. glabrata susceptibility develop during caspofungin therapy. We report a 68-year-old African-American female with a history of chronic kidney disease, diabetes, hypertension, restrictive lung disease, cerebrovascular disease, bilateral deep vein thrombosis, and a seizure disorder who developed shortness of breath over a 2-day period. She experienced a cardiopulmonary arrest status postintubation for worsening hypoxemia. Her hospital course was complicated by multiple nosocomial infections, including disseminated candidiasis.
During her first candidemic episode (sample 06-3168), she received caspofungin at a 70-mg loading dose and then at 50 mg/day. During the 14-day course, she experienced acute renal failure secondary to sepsis-induced hypotension but ultimately resolved her signs and symptoms. During evaluation of a new febrile episode 5 days after the completion of antifungal therapy, a femoral hemodialysis catheter tip culture revealed C. glabrata (sample 06-3169). Abdominal computerized tomography also revealed an undrainable pelvic abscess secondary to poor surgical candidacy. Caspofungin was again administered successfully. Two days after antifungal pharmacotherapy, she was again febrile with hypotension, and C. glabrata (sample 06-3170) was again recovered from peripheral blood culture. A third course of caspofungin was administered. The central intravenous catheters were removed and replaced in new positions after the first and during the third infection. However, the femoral hemodialysis catheter was changed over a guide wire and was not removed after the second infection. A fatal cardiopulmonary arrest occurred on day 11 of this relapse.
Although autopsy did not identify any previously unrecognized problems, the antifungal failure led to the suspicion of acquired drug resistance. Antifungal susceptibility tests were performed using a modified M27-A2 method described by Pfaller et al. (12, 16). The initial strain showed low MICs similar to those for the reference C. glabrata strain (ATCC 90030) for all three echinocandin drugs (caspofungin MIC, 0.06 μg/ml; micafungin MIC, 0.06 μg/ml; and anidulafungin MIC, 0.03 μg/ml), while the two follow-up isolates displayed high MICs (>2 μg/ml), consistent with reduced echinocandin susceptibility. All three strains displayed comparable susceptibilities to the triazoles fluconazole (1 to 2 μg/ml) and voriconazole (0.03 μg/ml) as well as to amphotericin B (0.125 μg/ml), suggesting pharmacotherapy-specific elevations in the MICs. All isolates were identified as C. glabrata by amplification and sequencing of the 5.8S RNA gene and adjacent internal transcribed spacers (ITS1 and ITS2). Moreover, multilocus sequence type analysis was done as previously described (2) to establish that the strains were clonally related (data not shown).
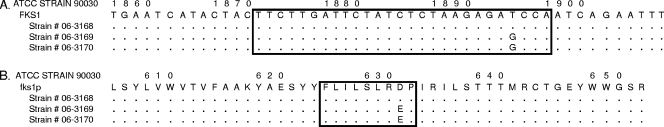
Echinocandin resistance in Candida spp. has been linked to amino acid changes in two highly conserved regions of glucan synthase subunits Fks1p and Fks2p (15). To assess these characteristic genetic changes, DNAs were extracted (Q-Biogene [Irvine, CA] Fast-DNA kit) from the three isolates and from control isolates following 16 h of growth in yeast extract-peptone-dextrose broth. C. glabrata FKS1 (GenBank accession no. XM_446406) was amplified and sequenced from nucleotides (nt) 1600 to 2513 and from nt 3448 to 4796. On the other hand, FKS2 (GenBank accession no. XM_448401) was amplified and sequenced from nt 1651 to 2225 and from nt 3749 to 4397. The susceptible C. glabrata strain contained wild-type sequences in the hot spot regions of FKS1 and FKS2, while both strains with reduced susceptibilities (06-3169 and 06-3170) contained a T1896G mutation that conferred a D632E substitution in Fks1p (Fig. 1). This amino acid is equivalent to D648 in C. albicans Fks1p, which is part of hot spot 1 (13). This hot spot 1 modification is consistent with previously reported echinocandin resistance in C. glabrata, although in that case an FKS2 mutation was implicated (7).
FIG. 1.
(A) Alignment of C. glabrata fks1 gene sequences showing the mutation in hot spot 1 (square). Dots represent the same nt as those in the reference strain (90030). (B) Alignment of C. glabrata Fks1p sequences showing the amino acid change. The square shows C. glabrata Fks1p hot spot 1.
The development of acquired resistance during therapy has rarely been reported (4, 9, 10). Alterations in C. albicans susceptibility were observed after long-term micafungin therapy in a human immunodeficiency virus-infected patient with chronic esophagitis. DNA sequencing demonstrated the appearance of characteristic FKS1 mutations (9). In a separate but similar case, therapeutic failure and decreasing susceptibilities of four clonal isolates of C. albicans were explored. Again, sequencing of FKS1 revealed a mutation encoding an amino acid change at F641 (1, 13).
Caspofungin resistance has rarely been reported for short-term pharmacotherapy of disseminated disease. Despite this, an acute myeloid leukemic patient with severe oropharyngeal candidiasis due to C. krusei developed endophthalmitis while undergoing a 2-week caspofungin treatment. A C. krusei mouth isolate obtained late in therapy was found to have a high MIC and appeared to be related clonally to an earlier bloodstream isolate (4). Thereafter, this resistant isolate was demonstrated to have a heterozygous P655C amino acid change in Fks1p hot spot 1, explaining the echinocandin resistance phenotype (6). In the second C. krusei case, reduced susceptibility (MIC = 2 μg/ml) was demonstrated for a case of disseminated disease. Unfortunately, clonality and FKS1/2 mutations were not assessed (14). In the final two cases, i.e., one case of a prosthetic aortic heart valve infection with C. parapsilosis and one case of C. glabrata candidemia, long courses of therapy (>6 weeks) were administered prior to identification of caspofungin resistance (8, 11). Clonality was demonstrated for the four isolates collected from one patient with candidemia (8).
Echinocandin class inhibitors modify the cell wall and are highly effective antifungal agents that exert strong selection pressure for reduced susceptibility. Owing to the rapid development of reduced susceptibility during this patient's hospitalization, clinicians should be diligent in monitoring patients for therapeutic failure secondary to these observed changes.
Acknowledgments
J.C. and S.C. have been consultants to Pfizer, Enzon, Astellas, Merck, and Three Rivers Pharmaceutics in addition to receiving research grants from all of these manufacturers. J.C. has received federal grants for the development of an amphotericin formulation and is working with Cumberland Pharmaceuticals to develop this product. D.P. and G.G. certify that they do not have an association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). Financial support for this work was provided by Pfizer Pharmaceuticals.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Baixench, M. T., N. Aoun, M. Desnos-Ollivier, et al. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076-1083. [DOI] [PubMed] [Google Scholar]
- 2.Dodgson, A. R., C. Pujol, D. W. Denning, D. R. Soll, and A. J. Fox. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J. Clin. Microbiol. 41:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst, E. J., J. G. Miller, R. Peson, M. J. Klevay, M. Pfaller, and D. J. Diekema. 2006. Risk factors for C. glabrata bloodstream infection: a case control study, abstr. M897. Abstr. 18th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Hakki, M., J. F. Staab, and K. A. Marr. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson, M. D., and J. R. Perfect. 2003. Caspofungin: first approved agent in a new class of antifungals. Expert Opin. Pharmacother. 4:807-823. [DOI] [PubMed] [Google Scholar]
- 6.Kahn, J. N., G. Garcia-Effron, M. J. Hsu, S. Park, K. A. Marr, and D. S. Perlin. 2007. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob. Agents Chemother. 51:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938-944. [DOI] [PubMed] [Google Scholar]
- 9.Laverdiere, M., R. G. Lalonde, J. G. Baril, D. C. Sheppard, S. Park, and D. S. Perlin. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705-708. [DOI] [PubMed] [Google Scholar]
- 10.Miller, C. D., B. W. Lomaestro, S. Park, and D. S. Perlin. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877-880. [DOI] [PubMed] [Google Scholar]
- 11.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Ap-proved standard M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 13.Park, S., R. Kelly, J. N. Kahn, et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelletier, R., I. Alarie, R. Lagace, and T. J. Walsh. 2005. Emergence of disseminated candidiasis caused by Candida krusei during treatment with caspofungin: case report and review of literature. Med. Mycol. 43:559-564. [DOI] [PubMed] [Google Scholar]
- 15.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]