Abstract
Levels of apoptosis induction (4′,6′-diamidino-2-phenylindole staining, activation of caspase 3) for aminoglycosides were compared by using renal LLC-PK1 cells. Amikacin caused less apoptosis than gentamicin in incubated cells. In electroporated cells, neomycin B and gentamicin caused apoptosis in the 0.03 to 0.1 mM range, isepamicin required larger concentrations (0.2 mM), and amikacin was without effect.
Multiresistance in gram-negative bacteria (1, 11) and a lack of truly novel compounds (24) have led to calls for improvement of formerly established antibiotics. Aminoglycosides (7) offer possibilities in this context (16, 23, 26), but their nephrotoxic potential remains of concern (7). Aminoglycosides accumulate in kidney proximal tubular cells by receptor-mediated endocytosis (14, 19) and trigger a sequence of alterations that include apoptosis (4, 13, 25). Gentamicin-induced apoptosis can be reproduced with cultured renal LLC-PK1 cells (3, 5, 9), either by incubating them with large drug concentrations or by electroporating them at low concentrations (20). Amikacin, which resists inactivation by several aminoglycoside-modifying enzymes (12), has been shown to cause less renal apoptosis than gentamicin in animals treated at therapeutically relevant doses (4, 8, 10). In the present study, we have examined whether amikacin can also be differentiated from gentamicin for apoptosis by using incubated and electroporated cells. In the latter model, we included neomycin B (a well-known nephrotoxic aminoglycoside) (7) and isepamicin (which shares many of the properties of amikacin, including its lower potential for nephrotoxicity compared to gentamicin) (13, 15).
All methods and products were as previously described (20, 22) except for minor modifications (see the supplemental material). Cell-associated aminoglycosides were measured by a microbiological technique (20; linear response for both gentamicin and amikacin [R2 > 0.99]). All aminoglycosides were obtained as pure compounds (microbiological standards from the original manufacturer) or purchased from Sigma-Aldrich or Serva Fine Chemicals GmbH (Heidelberg, Germany). Gentamicin and amikacin were also obtained as the products registered for clinical use in Belgium. All concentrations are expressed as free base (see the supplemental material for structures with molecular weights). Statistical analyses were made using GraphPad Prism version 4.02 and GraphPad InStat version 3.06 (GraphPad Prism Software, San Diego, CA).
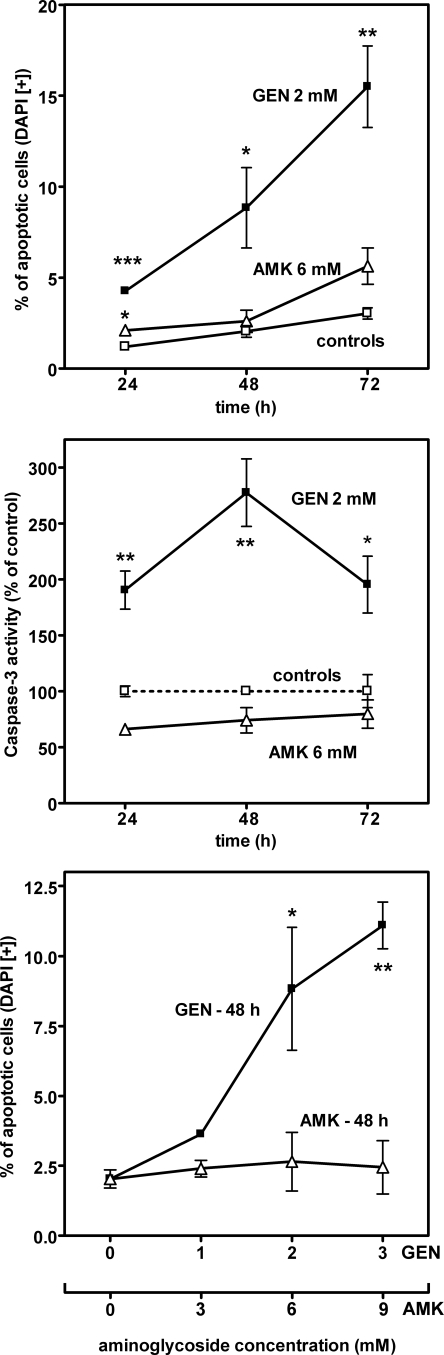
Figure 1 shows data obtained with cells incubated with gentamicin or amikacin. Gentamicin (2 mM [926 mg/liter]) caused a marked, time-dependent increase in the percentage of apoptotic cells (as in reference 5), whereas amikacin (6 mM [3.516 g/liter]) was without effect at days 1 and 2 and caused only a small increase at day 3. Gentamicin also caused a marked increase in caspase 3 activity at day 1, followed by a maximum at day 2 and a decrease thereafter. Caspase 3 activity in cells incubated with amikacin was slightly lower than or similar to that of controls. Apoptosis, measured after 2 days of incubation, proceeded in a concentration-dependent manner with gentamicin (0 to 3 mM [0 to 1.389 g/liter]), whereas amikacin was without a significant effect at concentrations of up to 9 mM (5.274 g/liter). The accumulations of both drugs measured at 48 h were linearly related to their extracellular concentrations, with slopes of 11.9 ± 0.9 nmol·mg of protein−1·mM−1 for gentamicin and 7.68 ± 0.51 for amikacin. As a result, cells incubated with amikacin had actually a 1.9-fold-larger drug molar content than those incubated with gentamicin when the values were compared at an extracellular concentration molar ratio of 3:1 (corresponding to their most common dosage ratios for humans, which are 4 mg/kg [8.56 μmol/kg] for gentamicin and 15 mg/kg [25.6 μmol/kg] for amikacin). Lactate dehydrogenase release (index of necrosis [20]) remained nonsignificantly different from that from the matching controls under all conditions.
FIG. 1.
Percentage of apoptotic cells (upper and lower panels) and increase of caspase 3 activity (middle panel) in LLC-PK1 cells incubated in the absence of aminoglycoside (controls; open squares) or in the presence of gentamicin (GEN; closed squares) or amikacin (AMK; open triangles). Upper panel, cells were incubated for up to 3 days without or with 2 mM gentamicin (0.926 g/liter) or 6 mM amikacin (3.51 g/liter), and the percentage of apoptotic nuclei was determined by microscopic examination after 4′,6′-diamidino-2-phenylindole (DAPI) staining (14.3 μM camptothecin [5 mg/liter] was used as the positive control (22) and yielded values of 43.1% ± 1.8%, 40.8% ± 2.2%, and 48.7% ± 2.8% of control values at 24, 48, and 72 h, respectively). Middle panel, same conditions of incubation as in the upper panel. Caspase 3 activity was assayed by using N-acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (see the supplemental material). Cells incubated with camptothecin yielded values of 2,767% ± 213%, 653% ± 41%, and 150% ± 35% of control values at 24, 48, and 72 h, respectively. Lower panel, cells were incubated for 48 h without aminoglycoside or with gentamicin (1 to 3 mM; 0.463 to 1.39 g/liter) or amikacin (3 to 9 mM; 1.76 to 5.26 g/liter). All values are means ± standard deviations (n = 3). Statistical analysis (two-tailed analysis of variance) for differences between treated cells and matched controls (upper and middle panels) or between cells incubated with and without aminoglycoside (lower panel): *, P < 0.05; **, P < 0.01; ***, P < 0.001. All comparisons between gentamicin and amikacin are made at a 1:3 molar ratio to correspond to the daily dosage ratios of these drugs for common therapeutic applications (see the text).
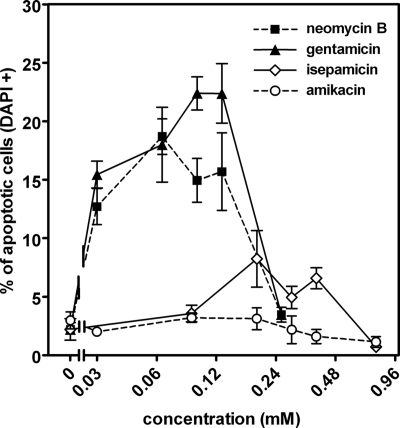
In the next series of experiments, cells were electroporated in the presence of increasing concentrations of neomycin B, gentamicin, isepamicin, or amikacin. As shown in Fig. 2, neomycin B and gentamicin caused a marked increase in apoptosis for concentrations (during electroporation) spanning between 0.032 and 0.128 mM, with a maximum at 0.064 mM (39.2 mg/liter) for neomycin B and at around 0.1 mM (46.7 mg/liter) for gentamicin (the bell-shaped curve of apoptosis versus concentration is due to the development of necrosis once the concentration reaches a critical threshold; see reference 20 for a discussion). Isepamicin showed a considerably less-marked effect and larger concentrations (between 0.192 and 0.384 mM [109 to 218 mg/liter]) were required. Amikacin was without effect at all concentrations tested (results similar to those described here were obtained with the clinical forms of gentamicin and amikacin; see the supplemental material). The apparent cell concentrations for gentamicin and amikacin were determined 1 h after electroporation and were linearly related to their extracellular concentrations (R2 > 0.992) but with a larger slope for amikacin than for gentamicin (53.3 ± 1.7 versus 26.7 ± 1.5 nmol·mg of protein−1·mM−1 [P < 0.001]; the slope for gentamicin was similar to that previously reported [20]).
FIG. 2.
Apoptosis in electroporated cells. Cells were electroporated in the absence (controls) or in the presence of neomycin B, gentamicin, isepamicin, or amikacin and returned to aminoglycoside-free medium, and apoptotic nuclei were enumerated 24 h later. Values are means ± standard deviations (n = 3). Statistical analysis was performed by two-tailed analysis of variance (P < 0.01). All values for neomycin B and gentamicin, except those observed for the largest concentration tested (0.256 mM), are significantly different from those of the controls; isepamicin values observed for 0.192, 0.288, and 0.384 mM concentrations are significantly different from those of controls; amikacin values did not differ from control values. The 0.12 mM concentration corresponds to approximately 74 mg/liter for neomycin B, 56 mg/liter for gentamicin (taking into account the respective contents of the commercial gentamicin in C1, C1a, and C2 components), 68 mg/liter for isepamicin, and 70 mg/liter for amikacin. See the supplemental material for structures of tested compounds.
The present study extends to cultured and electroporated cells our observations made with rats, which showed that amikacin induces less apoptosis than gentamicin when tested at clinically relevant dosages (4). Under our culture conditions, LLC-PK1 cells take up aminoglycosides slowly and to a limited extent (20, 22), making it necessary to use extracellular concentrations that largely exceed those observed for blood in vivo. Electroporation, a method now widely used for gene transfer and drug delivery in the cytosol of eukaryotic cells without loss of viability (6), makes it possible (i) to compare drugs at more clinically relevant concentrations (the percentage of apoptotic cells being already about sevenfold larger than in controls for a gentamicin concentration as low as 0.03 mM [approximately 14 mg/liter]); (ii) to confirm the low apoptogenic potential of amikacin in comparison with gentamicin, while demonstrating that it is not related to a lower drug accumulation. The common behaviors of neomycin B and gentamicin, on one hand, and of amikacin and isepamicin, on the other hand, suggest specific interactions of these drugs with those intracellular constituents that are susceptible to the triggering of apoptosis (18, 20, 22). These should be further explored though systematic structure-activity relationship studies, but it already appears that the number of ionizable groups (and perhaps also their positions) could be critical (see the supplemental material).
Apoptosis is an established mechanism of renal drug-induced toxicity (21) that develops at lower dosages than necrosis (2, 4, 17). Although the renal toxicity of aminoglycosides may involve mechanisms other than apoptosis (7, 21), making clinically pertinent drug ranking quite complex, the method developed here may help in further refining approaches toward the selection of safer derivatives. Generally speaking, it may also prove useful for the study of other drugs which, under normal conditions, would only slowly or poorly reach their intracellular pharmacological or toxicological target.
Supplementary Material
Acknowledgments
Marie-Claire Cambier provided dedicated technical assistance for cell culture, as did Martial Vergauwen and Vincent Rucchin for the apoptosis studies.
F.V.B. is Maître de Recherches of the Belgian Fonds de la Recherche Scientifique (FRS-FNRS). This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grant nos. 2.4.601.06 and 3.4.597.06), the Action de Recherches Concertées of the Université Catholique de Louvain (2007-2012), and the Belgian Federal Science Policy Office (research projects P5/33 and P6/19).
Footnotes
Published ahead of print on 7 April 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Anonymous. 1999. Tackling antimicrobial resistance. Drug Ther. Bull. 37:9-16. [DOI] [PubMed] [Google Scholar]
- 2.Arany, I., and R. L. Safirstein. 2003. Cisplatin nephrotoxicity. Semin. Nephrol. 23:460-464. [DOI] [PubMed] [Google Scholar]
- 3.Choi, K. H., T. I. Kim, D. L. Chong, H. Y. Lee, and D. S. Han. 2000. Gentamicin induced apoptosis of renal tubular epithelial (LLC-PK1) cells. Korean J. Intern. Med. 15:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Mouedden, M., G. Laurent, M. P. Mingeot-Leclercq, H. S. Taper, J. Cumps, and P. M. Tulkens. 2000. Apoptosis in renal proximal tubules of rats treated with low doses of aminoglycosides. Antimicrob. Agents Chemother. 44:665-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Mouedden, M., G. Laurent, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2000. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol. Sci. 56:229-239. [DOI] [PubMed] [Google Scholar]
- 6.Gehl, J. 2003. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 177:437-447. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert, D. N. 2005. Aminoglycosides, p. 328-356. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Elsevier/Churchill Livingstone, Philadelphia, PA.
- 8.Hottendorf, G. H., and L. L. Gordon. 1980. Comparative low-dose nephrotoxicities of gentamicin, tobramycin, and amikacin. Antimicrob. Agents Chemother. 18:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juan, S. H., C. H. Chen, Y. H. Hsu, C. C. Hou, T. H. Chen, H. Lin, Y. L. Chu, and Y. M. Sue. 2007. Tetramethylpyrazine protects rat renal tubular cell apoptosis induced by gentamicin. Nephrol. Dial. Transplant. 22:732-739. [DOI] [PubMed] [Google Scholar]
- 10.Laurent, G., M. B. Carlier, B. Rollman, F. Van Hoof, and P. Tulkens. 1982. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem. Pharmacol. 31:3861-3870. [DOI] [PubMed] [Google Scholar]
- 11.Livermore, D. M. 2003. Bacterial resistance: origins, epidemiology, and impact. Clin. Infect. Dis. 36:S11-S23. [DOI] [PubMed] [Google Scholar]
- 12.Mingeot-Leclercq, M. P., Y. Glupczynski, and P. M. Tulkens. 1999. Aminoglycosides: activity and resistance. Antimicrob. Agents Chemother. 43:727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mingeot-Leclercq, M. P., and P. M. Tulkens. 1999. Aminoglycosides: nephrotoxicity. Antimicrob. Agents Chemother. 43:1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moestrup, S. K., S. Cui, H. Vorum, C. Bregengard, S. E. Bjorn, K. Norris, J. Gliemann, and E. I. Christensen. 1995. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J. Clin. Investig. 96:1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rankin, L. I., F. C. Luft, M. N. Yum, R. S. Sloan, C. B. Dinwiddie, Jr., and L. L. Isaacs. 1979. Comparative nephrotoxicity of SCH 21420 and amikacin in rats. Antimicrob. Agents Chemother. 16:491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu, D. H., A. Litovchick, and R. R. Rando. 2002. Stereospecificity of aminoglycoside-ribosomal interactions. Biochemistry 41:10499-10509. [DOI] [PubMed] [Google Scholar]
- 17.Saboliæ, I. 2006. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol. 104:107-114. [DOI] [PubMed] [Google Scholar]
- 18.Sandoval, R. M., and B. A. Molitoris. 2004. Gentamicin traffics retrograde through the secretory pathway and is released in the cytosol via the endoplasmic reticulum. Am. J. Physiol. Renal Physiol. 286:F617-F624. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz, C., J. Hilpert, C. Jacobsen, C. Boensch, E. I. Christensen, F. C. Luft, and T. E. Willnow. 2002. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 277:618-622. [DOI] [PubMed] [Google Scholar]
- 20.Servais, H., Y. Jossin, F. Van Bambeke, P. M. Tulkens, and M. P. Mingeot-Leclercq. 2006. Gentamicin causes apoptosis at low concentrations in renal LLC-PK1 cells subjected to electroporation. Antimicrob. Agents Chemother. 50:1213-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Servais, H., A. Ortiz, O. Devuyst, S. Denamur, P. M. Tulkens, and M.-P. Mingeot-Leclercq. 2008. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 13:11-32. [DOI] [PubMed] [Google Scholar]
- 22.Servais, H., P. Van Der Smissen, G. Thirion, G. Van der Essen, F. Van Bambeke, P. M. Tulkens, and M.-P. Mingeot-Leclercq. 2005. Gentamicin-induced apoptosis in LLC-PK1 cells: involvement of lysosomes and mitochondria. Toxicol. Appl. Pharmacol. 206:321-333. [DOI] [PubMed] [Google Scholar]
- 23.Silva, J. G., and I. Carvalho. 2007. New insights into aminoglycoside antibiotics and derivatives. Curr. Med. Chem. 14:1101-1119. [DOI] [PubMed] [Google Scholar]
- 24.Talbot, G. H., J. Bradley, J. E. Edwards, Jr., D. Gilbert, M. Scheld, and J. G. Bartlett. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 25.Tulkens, P. M. 1986. Experimental studies on nephrotoxicity of aminoglycosides at low doses. Mechanisms and perspectives. Am. J. Med. 80:105-114. [DOI] [PubMed] [Google Scholar]
- 26.Yang, G., J. Trylska, Y. Tor, and J. A. McCammon. 2006. Binding of aminoglycosidic antibiotics to the oligonucleotide A-site model and 30S ribosomal subunit: Poisson-Boltzmann model, thermal denaturation, and fluorescence studies. J. Med. Chem. 49:5478-5490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.