Abstract
Aplaviroc (AVC), an experimental CCR5 inhibitor, potently blocks in vitro the infection of R5-tropic human immunodeficiency virus type 1 (R5-HIV-1) at subnanomolar 50% inhibitory concentrations. Although maraviroc is presently clinically available, further studies are required to determine the role of CCR5 inhibitors in combinations with other drugs. Here we determined anti-HIV-1 activity using combinations of AVC with various anti-HIV-1 agents, including four U.S. Food and Drug Administration-approved drugs, two CCR5 inhibitors (TAK779 and SCH-C) and two CXCR4 inhibitors (AMD3100 and TE14011). Combination effects were defined as synergistic or antagonistic when the activity of drug A combined with B was statistically greater or less, respectively, than the additive effects of drugs A and A combined and drugs B and B combined by using the Combo method, described in this paper, which provides (i) a flexible choice of interaction models and (ii) the use of nonparametric statistical methods. Synergistic effects against R5-HIV-1Ba-L and a 50:50 mixture of R5-HIV-1Ba-L and X4-HIV-1ERS104pre (HIV-1Ba-L/104pre) were seen when AVC was combined with zidovudine, nevirapine, indinavir, or enfuvirtide. Mild synergism and additivity were observed when AVC was combined with TAK779 and SCH-C, respectively. We also observed more potent synergism against HIV-1Ba-L/104pre when AVC was combined with AMD3100 or TE14011. The data demonstrate a tendency toward greater synergism with AVC plus either of the two CXCR4 inhibitors compared to the synergism obtained with combinations of AVC and other drugs, suggesting that the development of effective CXCR4 inhibitors may be important for increasing the efficacies of CCR5 inhibitors.
CCR5 is a member of the G-protein-coupled, seven-transmembrane-segment receptors, which comprise the largest superfamily of proteins in the body (30). In 1996, it was revealed that CCR5 serves as one of the two essential coreceptors for the entry of human immunodeficiency virus type 1 (HIV-1) into human CD4+ cells, thereby serving as an attractive target for possible interventions against HIV-1 infection (1, 9, 40, 42). Consequently, scores of small-molecule CCR5 inhibitors which exert potent activity against R5-tropic HIV-1 (R5-HIV-1) were identified (2, 10, 19, 35). Aplaviroc (AVC), a spirodiketopiperazine derivative, represents one such experimental small-molecule CCR5 inhibitor (17, 18). AVC binds to human CCR5 with a high affinity, blocks HIV-1 gp120 binding to CCR5, and exerts potent activity against a wide spectrum of laboratory and primary R5-HIV-1 isolates, including multidrug-resistant HIV-1 isolates (50% inhibitory concentrations, 0.2 to 0.6 nM) (17, 18). Maraviroc (MVC) is another small-molecule CCR5 inhibitor which has become the first CCR5 inhibitor approved for the treatment of AIDS and HIV-1 infection by the U.S. Food and Drug Administration (FDA). One possible concern over the long-term use of CCR5 inhibitors is the change of viral tropism, which enables the virus to use the CXCR4 receptor (20, 41); therefore, CCR5 inhibitors are unlikely to be used as single agents. Assessments of the interaction of CCR5 inhibitors with other anti-HIV-1 agents should thus help provide an understanding of the role of CCR5 inhibitors and help design regimens to be used for the treatment of individuals infected with HIV-1.
In the present study, we determined the effects against R5-HIV-1Ba-L of AVC in combination with various anti-HIV-1 agents which affect other steps of the viral life cycle, including a nucleoside reverse transcriptase inhibitor, zidovudine (ZDV); a nonnucleoside reverse transcriptase inhibitor, nevirapine (NVP); a protease inhibitor, indinavir (IDV); and a fusion inhibitor, enfuvirtide (ENF). We assessed the synergistic effects of AVC in combination with CXCR4 inhibitors as well as the other drugs described above against a mixture of R5-HIV-1Ba-L and X4-HIV-1ERS104pre (designated HIV-1Ba-L/104pre). In the present study, we also developed an evaluation system, designated the Combo method, which provides (i) a flexible choice of interaction models, (ii) the use of nonparametric statistical methods to obtain P values for comparison, and (iii) flexibility with respect to experimental design (e.g., checkerboard and constant-ratio designs). The present data suggest that AVC exerts antiviral synergy when it is used with other classes of anti-HIV-1 agents but apparently not when it is used with other CCR5 inhibitors. The present data also demonstrate a tendency toward greater synergism with AVC plus either of the two CXCR4 inhibitors examined in comparison to the synergism obtained with combinations of AVC and other FDA-approved drugs, suggesting that the development of effective CXCR4 inhibitors may be important for increasing the efficacies of CCR5 inhibitors.
MATERIALS AND METHODS
Antiviral agents.
AVC is an experimental CCR5 inhibitor containing a spirodiketopiperazine core, as described previously (18, 19, 26). TAK779, SCH-C, and AMD3100 were synthesized as described previously (2, 7, 35). ZDV was purchased from Sigma (St. Louis, MO). IDV was kindly provided by Japan Energy Inc. (Tokyo, Japan). TE14011 and ENF were synthesized as described previously (36, 37). NVP was a kind gift from Boehringer Ingelheim Pharmacerticals Inc. (Ridgefield, CT).
Viruses.
R5-HIV-1Ba-L was obtained from the AIDS Research and Reference Reagent Program (13). X4-HIV-1ERS104pre was isolated from a drug-naïve patient with AIDS (33). These HIV-1 isolates were propagated in phytohemagglutinin-stimulated peripheral blood mononuclear cells (PHA-PBMCs), and the culture supernatants were harvested and stored at −80°C until use (22). In certain experiments, a 50:50 mixture of HIV-1Ba-L and HIV-1ERS104pre (HIV-1Ba-L/104pre) was prepared.
Assay for in vitro anti-HIV-1 activity.
PBMCs were isolated from the buffy coats of HIV-1-seronegative individuals by Ficoll-Hypaque density gradient centrifugation and were cultured at a concentration of 106 cells/ml in RPMI 1640-based culture medium supplemented with 10% fetal calf serum (FCS; HyClone Laboratories, Logan, UT), penicillin (50 U/ml), and streptomycin (50 μg/ml) (10% FCS-RPMI) with 10 μg/ml PHA for 3 days prior to the anti-HIV-1 activity assay in vitro. PHA-PBMCs (106/ml) from a 3-day culture were resuspended in 10% FCS-RPMI containing 10 ng/ml interleukin-2 and plated into each well of 96-well microculture plates (105 per well). Each of the test compounds was added as a single agent or in combination with another agent to each well of the microculture plates. For assessment of the effects of a combination of any two drugs, three threefold serial concentrations were chosen on the basis of the dose-response curve at which the percent inhibition values increased linearly.
The cells were subsequently exposed to 50 50% tissue culture infectious doses (TCID50s) of HIV-1Ba-L or a mixture of 25 TCID50s of HIV-1Ba-L and 25 TCID50s of HIV-1ERS104pre and incubated at 37°C in humidified air containing 5% CO2. On day 7 of culture, the cell-free culture supernatants were harvested and the HIV-1 p24 antigen levels in the supernatants were determined with a fully automated chemiluminescent enzyme immunoassay system (Lumipulse F; Fujirebio Inc., Tokyo, Japan) (18, 23). All the assays were performed in duplicate, and each experiment was conducted on 5 to 10 different occasions. No cytotoxicity was observed at the highest concentrations of each agent, as assessed by the trypan blue dye exclusion method.
Mathematical analysis: the Combo method.
We assessed the effects of drug combinations using the combination index (CI), calculated with CalcuSyn software (BioSoft, Cambridge, United Kingdom), which was based on the median-effect method developed by Chou and Talalay (3, 4). For experiments with combinations of the same drug, serially diluted drug concentrations were chosen on the basis of the 50% effective concentrations (EC50s), and each drug was combined with itself at the same concentration. As in the original method, CIs of <1, 1, and >1 were judged to represent synergism, additivity, and antagonism, respectively.
It should be noted that the Chou and Talalay median-effect method (3, 4) alone does not allow us to statistically compare the effects of the combinations. Thus, we devised a new method for evaluation of the effects of drug combinations, designated the Combo method. For the Combo method used in the present study, we used three concentrations of one drug (drug A) and three concentrations of the other drug (drug B) and combined the drugs at three different concentrations, preparing nine (3 × 3) combination cultures, and we obtained nine determinations of HIV-1 p24 concentrations (each combination assay was performed in duplicate). More precisely, three combinations were examined: the same drug A combination (drug A and drug A), the same drug B combination (drug B and drug B), and the combination of drug A and drug B. A full view of the data obtained with the drug combinations can be visualized (as shown in the Results section) in three-dimensional (3-D) figures by the use of Microsoft Excel software (version 11.0, 2004; Microsoft Corporation, Redmond, WA), based on the method of Prichard and colleagues (27, 28, 29). It is of note that with the Bliss independence method, the predicted additive effects at each combination point are subtracted from the inhibitory effects of the combination determined from the experimental drug combination assay, generating percent synergy values, and the points plotted above the predicted additive effects represent synergism, while the points below the plane represent antagonism. Using the Bliss independence method, we calculated percent synergy values for the nine determinations described above, and the average value was further computed, generating a mean percent synergy value (%synergymean). We repeated this assay for each drug combination 5 or 10 times on different occasions. These 5 or 10 %synergymean values thus obtained for a set of combinations (drug A-drug A, drug B-drug B, and drug A-drug B) were compared with the other data sets (5 or 10 %synergymean values) by the Wilcoxon rank sum test, generating P values for each combination set. All P values are two-tailed and have not been formally adjusted for multiple comparisons. However, in the context of the several experiments and comparisons performed, P values of <0.01 would clearly indicate statistical significance, while differences with values of 0.01 < P < 0.05 would indicate strong trends.
RESULTS
Activities of anti-HIV-1 agents in PHA-PBMCs.
We first determined the antiviral potencies of seven anti-HIV-1 agents (AVC, SCH-C, TAK779, ZDV, NVP, IDV, and ENF) against HIV-1Ba-L employing PHA-PBMCs as target cells (Table 1). AVC had a potent inhibitory effect against HIV-1Ba-L, with mean EC50, EC75, EC90, and EC95 values of 0.7, 4, 16, and 25 nM, respectively. SCH-C and TAK779, which are both CCR5 inhibitors, also showed potent antiviral activity (but with less potent antiviral activity compared to that of AVC), with EC50s of 6 and 20 nM, respectively. To determine the additive effects of AVC-AVC and AMD3100-AMD3100, we employed R5-HIV-1Ba-L and X4-HIV-1ERS104pre as the virus inocula, respectively, since AVC is inert against X4-HIV-1 and AMD3100 is inert against R5-HIV-1. These two agents were found to be potent against the virus, with EC50s of 26 and 4 nM, respectively. No toxicity of any of the anti-HIV-1 agents was observed at concentrations up to 1.0 μM, as determined by examination of PHA-PBMCs (data not shown).
TABLE 1.
Anti-HIV-1 activity of each drug in the assay system
| Virus | Compound | EC (nM) for anti-HIV-1 activitya
|
|||
|---|---|---|---|---|---|
| 50% | 75% | 90% | 95% | ||
| Ba-L | AVC | 0.7 ± 0.4 | 4.0 ± 4.0 | 16 ± 15 | 25 ± 14 |
| SCH-C | 6.8 ± 6.0 | 31 ± 18 | 94 ± 43 | 131 ± 64 | |
| TAK779 | 20 ± 14 | 127 ± 83 | 332 ± 192 | 576 ± 224 | |
| ZDV | 18 ± 4.0 | 58 ± 3.0 | 128 ± 54 | 178 ± 49 | |
| NVP | 19 ± 2.0 | 36 ± 11 | 127 ± 39 | 149 ± 47 | |
| IDV | 29 ± 7.0 | 44 ± 12 | 75 ± 18 | 87 ± 13 | |
| ENF | 11 ± 4.0 | 46 ± 5.0 | 82 ± 14 | 98 ± 16 | |
| 104pre | AMD3100 | 26 ± 8.0 | 96 ± 21 | 193 ± 51 | 257 ± 46 |
| TE14011 | 4.0 ± 1.0 | 16 ± 7.0 | 50 ± 11 | 78 ± 17 | |
The EC50, EC75, EC90, and EC95 values were determined by using PHA-PBMCs isolated from three different donors and the inhibition of p24 Gag protein production as the end point. All assays were conducted in triplicate. The results shown represent the arithmetic means (±1 standard deviation) of the values from three independently conducted assays.
Same-drug combination and additivity.
To determine whether combinations of two different anti-HIV-1 agents produced synergistic, additive, or antagonistic effects, we first attempted to establish an algorithm so that the effects of the combination of the same drug (i.e., drug A-drug A) represent additivity. We determined the effects of combinations of the same drug for each of the seven anti-HIV-1 agents using the CIs dictated by the median-effect method (4). Figure 1A shows three representative dose-response curves of the percent inhibition of HIV-1 replication in the presence of a CCR5 inhibitor (AVC) alone, a reverse transcriptase inhibitor (ZDV) alone, a CXCR4 inhibitor (AMD3100) alone, or AVC plus AVC, ZDV, or AMD3100. A range of concentrations at which the percent inhibition values linearly increased was identified (Fig. 1A and B) and was used to examine the effects of any combination of two drugs chosen.
FIG. 1.
Dose-response curves of single and combined drug assays. Three representative dose-response curves are shown. (A) Dose-response curve with the same-drug combination (AVC-AVC). PHA-PBMCs were exposed to R5-HIV-1Ba-L and cultured in the presence of AVC alone or AVC-AVC over 7 days. AVC was serially diluted threefold to give concentrations in the range of 0.1 to 24.3 nM. The percent inhibition values were determined on the basis of the amounts of p24 Gag proteins in the culture supernatants. (B) AVC was combined with ZDV at a fixed ratio (1:11), and the assay was conducted as described above for panel A. (C) AVC (concentration range, 0.3 to 72.9 nM) was combined with AMD3100 at a 1:11 ratio. PHA-PBMCs were exposed to a 50:50 mixture of R5-HIVBa-L and X4-HIV-1ERS104pre and cultured in the presence of AVC alone, AMD3100 alone, or AVC-AMD3100. All assays were performed on 5 to 10 different occasions, and all the values shown represent the arithmetic means ± 1 standard deviation.
We found that the same-drug combination of AVC-AVC which gave a 50% reduction of HIV-1 replication produced a CI of 1.03 ± 0.09 (Table 2), indicating that this combination produced additivity on the basis of the median-effect method. However, that same-drug combination which gave 75, 90, and 95% reductions in viral replication produced CIs of 0.82, 0.71, and 0.68, respectively, which indicated that this same-drug combination produced synergistic effects. Synergistic effects were similarly indicated when the other anti-HIV-1 agents were examined as same-drug combinations in our analysis (Table 2).
TABLE 2.
CIs against HIV-1 obtained with mixtures of the same compounds at various inhibitory concentrations
| Virus | Combinationb | CIa
|
|||
|---|---|---|---|---|---|
| 50% | 75% | 90% | 95% | ||
| Ba-L | AVC + AVC | 1.03 ± 0.09 | 0.82 ± 0.10 | 0.71 ± 0.10 | 0.68 ± 0.09 |
| ZDV + ZDV | 1.08 ± 0.14 | 0.95 ± 0.18 | 0.84 ± 0.23 | 0.81 ± 0.22 | |
| NVP + NVP | 0.99 ± 0.09 | 0.81 ± 0.11 | 0.69 ± 0.12 | 0.66 ± 0.14 | |
| IDV + IDV | 1.02 ± 0.06 | 0.91 ± 0.05 | 0.79 ± 0.07 | 0.76 ± 0.06 | |
| ENF + ENF | 1.04 ± 0.08 | 0.89 ± 0.08 | 0.75 ± 0.09 | 0.73 ± 0.11 | |
| 104pre | AMD + AMD | 1.12 ± 0.12 | 0.88 ± 0.09 | 0.69 ± 0.09 | 0.67 ± 0.10 |
| TE + TE | 1.05 ± 0.15 | 0.90 ± 0.11 | 0.80 ± 0.13 | 0.78 ± 0.13 | |
Drug interactions of same-drug combinations were analyzed by using CIs. CIs were calculated on the basis of the model of Chou and Talalay (3, 4) with CalcuSyn software (BioSoft). Originally, CIs of <1, 1, or >1 indicated a synergistic effect, an additive effect, and antagonism, respectively. The drugs were combined at a 1:1 ratio, and all assays were conducted in duplicate. The results shown represent the arithmetic means (±1 standard deviation) of the CIs at various inhibitory concentrations (50%, 75%, 90%, and 95%) from 10 independently conducted assays.
AMD, AMD3100; TE, TE14011.
The indication of synergism in the same-drug combination described above was thought to be a limitation or error inherent to the median-effect method or to stem from the variability of the biological data obtained. Since the median-effect method does not provide room for statistical analysis or a full view of the combination data, we examined the same data set using Microsoft Excel software, based on the method of Prichard and colleagues (27, 28, 29), which gives a graphical 3-D view of the entire data set. In the analysis of the AVC-AVC combination data, this method with Microsoft Excel software indicated that the combination of the highest AVC concentration (2.7 nM AVC and 2.7 nM AVC) that produced synergism gave a percent synergy value of 2.2, although other combinations were determined to be additive or antagonistic, giving an average (± standard deviation) percent synergy value of −1.8 ± 2.4 (Fig. 2A). The same-drug combinations of ZDV, NVP, and ENF similarly gave partial synergism (Fig. 2B, C, and E). However, the same-drug combination of IDV indicated synergism with all data points, with an average percent synergy value of 3.6 ± 2.2 (Fig. 2D). We predicted that the partial synergism seen with AVC, ZDV, NVP, and ENF and the entire synergism seen with IDV also represented a limitation or error inherent to the method of Prichard and colleagues (27, 28, 29) or the variability of the biological data obtained.
FIG. 2.
Effects of same-drug combinations. The serially diluted anti-HIV-1 agents AVC (A), ZDV (B), NVP (C), IDV (D), and ENF (E) were combined with the same agent diluted under the same conditions; PHA-PBMCs were exposed to R5-HIV-1Ba-L and cultured in the presence of the drugs combined. The combination effects (percent synergy values on the vertical z axis) were determined on the basis of the Bliss independence method. In the 3-D graphs, obtained on the basis of the method of Prichard et al. (27, 28, 29), the average percent synergy values at each concentration derived from 10 experiments were plotted. The hatched area represents synergism (percent synergy values, >0), while the open area represents additivity or antagonism (percent synergy values, ≤0). Numbers in parentheses represent the average percent synergy values (±1 standard deviation). The x and y axes indicate the concentrations of the drug tested (nM). All assays were performed in duplicate, and each experiment was independently conducted 10 times.
AVC acts in synergy with ZDV, NVP, IDV, and ENF to block the replication of HIV-1Ba-L in PHA-PBMCs.
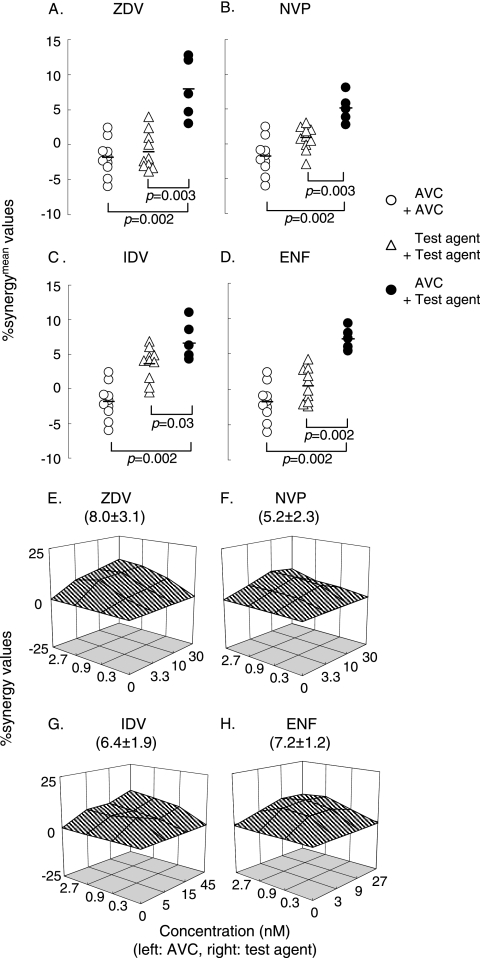
Considering that one of the main reasons for the partial synergism described above could stem from the variability of the cell-based assay data used in the present work, we used standard nonparametric statistical analysis methods to evaluate the differences. To this end, we conducted the drug-combination assay in duplicate and determined the %synergymean values in three settings: (i) drug A-drug A, (ii) drug B-drug B, and (iii) drug A-drug B. Experiments testing the drug A-drug A combination and the drug B-drug B combination were conducted on 10 different occasions, while the drug A-drug B combinations assay was conducted on 5 different occasions. As shown in Fig. 3A, as expected, the same-drug combination assays with AVC and ZDV produced relatively low average %synergymean values of −1.8 and −1.0, respectively. However, the AVC-ZDV combination gave a high average %synergymean value of 8.0. When we examined the difference among the AVC-AVC, ZDV-ZDV, and AVC-ZDV data using the Wilcoxon rank sum test, there was a statistically significant difference between the AVC-AVC and the AVC-ZDV data (P = 0.002) as well as between the ZDV-ZDV and the AVC-ZDV data (P = 0.003). The same was true when we examined the effects of NVP, IDV, and ENF in combination with AVC (Fig. 3B to D). With these data, we determined that if both the drug A-drug A and drug B-drug B combinations gave relatively low %synergymean values and a significant difference between the drug A-drug B combination and the same-drug combinations was detected, we would judge that there was significant synergism. When we plotted the average percent synergy value for the combination of drugs A and B at each different concentration on a point-by-point basis by the method of Prichard and colleagues (27, 28, 29), the results showed substantially higher levels of synergism for all data points (Fig. 3E to H). The average percent synergy values for AVC-ZDV, AVC-NVP, AVC-IDV, and AVC-ENF were 8.0 ± 3.1, 5.2 ± 2.3, 6.4 ± 1.9, and 7.2 ± 1.2, respectively, which corroborated the interpretation of the data shown in Fig. 3A to D. Thus, we interpreted that the addition of AVC to each of the other agents produced significant synergism.
FIG. 3.
Effects of AVC in combination with other anti-HIV-1 agents against R5-HIV-1Ba-L. Drug combination assays were conducted, and the %synergymean values (the mean of the nine percent synergy values from each set of the data) are shown in three settings: (i) AVC-AVC, (ii) test agent (to be combined with AVC)-test agent, and (iii) AVC-test agent (A to D). The AVC-AVC combination and the test agent-test agent combination were tested on 10 different occasions, while the AVC-test agent combination assay was done on 5 different occasions. The differences in the %synergymean values between the three settings were analyzed by using the Wilcoxon rank sum test. The short bars indicate the arithmetic means. The combination effects are also shown in 3-D graphs, as determined on the basis of the method of Prichard et al. (see the legend to Fig. 2).
Effects of AVC in combination with SCH-C or TAK779 against R5-HIV-1Ba-L.
We next asked whether AVC in combination with SCH-C or TAK779 had synergistic activity against HIV-1Ba-L (Fig. 4). The difference between the AVC-AVC and the AVC-SCH-C combinations was not statistically significant (P = 0.09), while there was evidence of a trend toward antagonism between the SCH-C-SCH-C and the AVC-SCH-C combinations (P = 0.05). It was interesting that when these data were examined by the method of Prichard and colleagues (27, 28, 29), a mixed pattern with an inclination toward antagonism was seen, with an average percent synergy of −4.4 ± 2.4. We also examined whether AVC had significant combination effects when it was combined with TAK779. There was a trend toward a statistically significant difference between AVC-AVC and AVC-TAK779 (P = 0.03) as well as TAK779-TAK779 and AVC-TAK779 (P = 0.05). However, when these data were plotted in the chart by the method of Prichard and colleagues (27, 28, 29), the pattern was a mixed one, with a low average percent synergy (1.6 ± 1.0), suggesting that synergism would be at a low level. However, it was noted that the same set of data for the combination of AVC and SCH-C produced CI values of 1.05 (at a 50% inhibitory effect) and 0.58 (at a 90% inhibitory effect), indicating that there was synergism between AVC and SCH-C, as analyzed on the basis of the median-effect method of Chou and Talalay (3, 4). It was thought that there was a propensity toward an overestimation of the combination effects toward synergism when the median-effect method was used.
FIG. 4.
Effects of AVC in combination with other CCR5 inhibitors. The effects of AVC in combination with SCH-C (A) or TAK779 (B) when they were exposed to R5-HIV-1Ba-L are shown. No significant synergism was seen when AVC was combined with SCH-C or TAK779 compared with that seen with AVC-AVC. There was a trend toward antagonism when AVC-SCH-C with SCH-C-SCH-C and a trend toward synergism when AVC-TAK779 was compared with TAK779-TAK779. When these data were examined by the method of Prichard et al. (27, 29), AVC-SCH-C showed a mixed pattern but with an inclination toward antagonism (C), while AVC-TAK779 showed a mixed pattern but with an inclination toward synergy (D).
Combination effects of AVC in a mixture of R5-HIV-1Ba-L and X4-HIV-1ERS104pre.
AVC exerts no antiviral activity against X4-HIV-1 (18, 23), although the HIV-1 population seen in individuals with HIV-1 infection often comprises both R5- and X4-HIV-1 populations. Hence, it would be reasonable to use a CCR5 inhibitor plus a CXCR4 inhibitor to treat individuals with HIV-1 infection (6). Thus, we attempted to examine effects of the combination of AVC and either AMD3100 and TE14011 against HIV-1Ba-L/104pre.
It is thought that the replication kinetics of HIV-1 strains tend to affect the results of any antiviral assay, in particular, when more than one HIV-1 isolate is employed in one assay. We therefore first conducted a set of experiments in order to delineate the replication curves for both the R5-tropic (HIV-1Ba-L) and X4-tropic (HIV-1ERS104pre) strains used in this study. It was confirmed that the two strains had comparable replication kinetics and that the p24 values of both strains were comparable over 7 days when the amount of each strain inoculated was adjusted on the basis of the TCID50 for the strain (data not shown). Moreover, the amounts of HIV-1 p24 produced by PBMCs that were exposed to the mixture of the R5- and X4-tropic strains and cultured in the presence of a high concentration of AVC were comparable to the amounts of HIV-1 p24 from PBMCs that were similarly treated but that were cultured in the presence of a high concentration of AMD3100 (Fig. 1C). These data suggested that HIV-1Ba-L and HIV-1ERS104pre replicate comparably in cell cultures inoculated with the 50:50 mixture of the viruses. To determine the additive effects of AVC-AVC and AMD3100-AMD3100, we employed R5-HIV-1Ba-L and X4-HIV-1ERS104pre as the target viruses, respectively, since AVC is inert against X4-HIV-1 and AMD3100 is inert against R5-HIV-1.
The AVC-AMD3100 combinations produced %synergymean values significantly different from those for AVC-AVC (P = 0.002) and those for AMD3100-AMD3100 (P = 0.005) (Fig. 5A). When these combination data were examined in the 3-D model of Prichard and colleagues (27, 28, 29), apparently high levels of synergism were seen for all data points, with an average percent synergy value of 8.0 ± 4.4 (Fig. 5G). When TE14011 was combined with AVC, synergism was similarly seen, with an average percent synergy value of 8.2 ± 4.5 (Fig. 5H). The %synergymean values for AVC-ENF were also greater than those for AVC-AVC (P = 0.005) and less than those for ENF-ENF (P = 0.04); however, when the level of synergism was examined in the 3-D model, it appeared to be relatively lower, with an average percent synergy value of 4.8 ± 4.2 (Fig. 5I).
FIG. 5.
Effects of AVC in combination with other anti-HIV-1 agents against a 50:50 mixture of R5-HIV-1Ba-L and X4-HIV-1ERS104pre. PHA-PBMCs were exposed to a 50:50 mixture of R5-HIV-1Ba-L and X4-HIV-1ERS104pre and cultured in the presence of AVC in combination with AMD3100 (A), TE14011 (B), ENF (C), ZDV (D), NVP (E), or IDV (F) for 7 days, and the amounts of p24 Gag proteins in the culture supernatants were determined. Differences in the %synergymean values between AVC-AVC, test agent-test agent, and AVC-test agent were examined by using the Wilcoxon rank sum test. A statistically significant difference, or a strong trend, was observed for all combinations except between IDV-IDV and AVC-IDV (P = 0.2). The short bars indicate the arithmetic means obtained. The average percent synergy values for the AVC-test agent combinations were also plotted in 3-D graphs, as determined on the basis of the method of Prichard et al. (see the legend to Fig. 2) (G to L). Assays with AVC in combination with each drug and the same-drug combination were performed 5 times and 10 times, respectively. All assays were conducted in duplicate.
We next examined the effect of AVC in combination with one of the three FDA-approved anti-HIV-1 agents, ZDV, NVP, and IDV. The %synergymean values obtained with AVC-ZDV or AVC-NVP were greater than those obtained with AVC-AVC, ZDV-ZDV, and NVP-NVP (P values for all comparisons, ≤0.005; Fig. 5D and E). In the 3-D model, synergism was also observed for ZDV and NVP in combination with AVC (Fig. 5J and K). AVC-IDV produced no significant difference in the %synergymean values compared to those for IDV-IDV (P = 0.2), although the effect of AVC-IDV was significantly different from the effect of AVC-AVC (Fig. 5F), and a substantial level of percent synergy was also seen in the 3-D model (Fig. 5L).
It was apparent that the %synergymean values of AVC-AMD3100 and AVC-TE14011 were greater than those of AVC plus any of the four antiviral agents (ENF, ZDV, NVP, and IDV). We therefore examined whether the apparent differences were statistically significant using the Wilcoxon rank sum test. The %synergymean values for AVC-AMD3100 were greater than those for AVC-ZDV (P = 0.0472) and AVC-NVP (P = 0.0472) but not those for AVC-ENF (P = 0.1745) and AVC-IDV (P = 0.4647). The %synergymean values of AVC-TE14011 were not statistically different from those of AVC plus any of the four agents (P > 0.05). Thus, even on the basis of a limited number of experiments, there were cases in which AVC-AMD3100 produced statistically greater synergism compared with the levels of synergism obtained with combinations of AVC and other conventional drugs. However, given the fact that the methodology used in the present study may as yet produce overestimates of synergy and the variability of the responses obtained by comparison of the combinations may also affect the analysis, it should be stressed that the effects of combinations of another CCR5 inhibitor(s) with another CXCR4 inhibitor(s) should be examined to confirm such synergism.
DISCUSSION
In the present study, we demonstrated that AVC exerts synergistic activity against HIV-1Ba-L in vitro when it is combined with ZDV, NVP, IDV, or ENF. These results are generally consistent with the data reported by Tremblay and colleagues, who examined two experimental CCR5 inhibitors, TAK220 and SCH-C, in combination with ZDV, lamivudine, IDV, efavirenz, or ENF for their effects against R5-HIV-1 isolates ( 38, 39).
Another CCR5 inhibitor, MVC, has recently received accelerated approval by the FDA for use in combination with other antiretroviral agents for the treatment of R5-HIV-1 in adults whose viral loads remain detectable despite existing antiviral treatment or who have multiple-drug-resistant viruses. In a recent 48-week data set from the MERIT study conducted with antiretroviral therapy-naïve subjects infected with R5-HIV-1 (32), 70.6% of the patients receiving MVC achieved HIV-1 RNA levels of less than 400 copies/ml, whereas 73.1% of the patients in the efavirenz group achieved HIV-1 RNA levels of less than 400 copies/ml, which met the criteria for noninferiority. However, when the HIV-1 RNA cutoff level of less than 50 copies/ml was used, noninferiority could not be confirmed. These data suggest that some of the patients who were determined to harbor R5-HIV-1 and who failed MVC therapy may have had X4-HIV-1, which MVC does not suppress.
It may be reasonable to suggest that R5-HIV-1 is not highly dominant in those infected with HIV-1. Indeed, the prevalence levels of R5- and X4-tropic viruses in patients with HIV-1 infection vary depending on the cohort examined. Demarest et al. demonstrated that the majority of drug-naïve and drug-experienced HIV-1-infected individuals harbored R5-HIV-1 (88% and 67%, respectively), while a mixture of R5- and X4-tropic viruses was seen in 12 and 28% of those individuals, respectively, and X4-tropic virus was seen in only 0 and 5% of those individuals, respectively (8). Fätkenheuer et al. have reported that the overall prevalence of R5-HIV-1 was 94% among the individuals whom they examined (11), while Daar et al. showed that 59.5% of 126 children and adolescents harbored R5-HIV-1 and the rest (40.5%) harbored viruses with dual or mixed tropisms (5). Taken together, the presence or absence of X4-HIV-1 in individuals who are to receive a CCR5 inhibitor, regardless of their positivity for X4-HIV-1, appears to be a critical factor for successful treatment with CCR5 inhibitor-containing regimens.
Using the Combo method (see below), we found that there were significant synergistic effects when AVC was combined with ZDV, NVP, IDV, or ENF and tested against HIV-1Ba-L/104pre (Fig. 5C to F). Interestingly, only a lower level of synergism was observed when AVC was combined with TAK779. When AVC was combined with SCH-C, no synergism was seen (Fig. 4A and C). In this regard, we have previously observed that the binding pockets for AVC, SCH-C, and TAK779 are all located in the same hydrophobic cavity within CCR5, although their binding profiles differ substantially from each other (17). We analyzed the interactions of these three inhibitors in relation to wild-type CCR5 (CCR5WT). When CCR5WT-overexpressed Chinese hamster ovarian cells were exposed to 3H-labeled AVC, followed by the addition of unlabeled SCH-C, 3H-labeled AVC binding to CCR5 was reduced only moderately. When the interaction between 3H-labeled AVC and unlabeled TAK779 was examined, 3H-labeled AVC binding to CCR5WT was not significantly replaced by unlabeled TAK779 binding. On the contrary, when 3H-labeled SCH-C was added first and then unlabeled AVC was added, the binding of 3H-labeled SCH-C to CCR5WT was significantly blocked, suggesting that AVC effectively replaced the 3H-labeled SCH-C and bound to CCR5WT. The binding of 3H-labeled TAK779 was likewise blocked by the addition of unlabeled AVC, although the extent of replacement of 3H-labeled TAK779 by AVC was less compared to the extent of replacement of 3H-labeled SCH-C by AVC. These results appear to be explained at least in part by the binding of these three inhibitors to the same hydrophobic cavity within CCR5, although their binding profiles are different from each other and their affinities of binding to CCR5WT are also different (17). Whatever the mechanism, the present data suggest that the combination of small-molecule CCR5 inhibitors does not seem to bring about synergistic activity and that caution should perhaps be used when the use of combinations of multiple CCR5 inhibitors is considered.
Notably, the most potent synergism was seen when AVC was combined with AMD3100 or TE14011, as examined against HIV-1Ba-L/104pre (Fig. 5A and B). The synergy values for AVC-AMD3100 and AVC-TE14011 were greater (8.0 ± 4.4 and 8.2 ± 4.5, respectively) than those for any other combination (Fig. 5G and H). Hirsch's group has also examined the effects of the combination of aminooxypentane-RANTES and a derivative of stroma-derived factor 1β, using a mixture of R5- and X4-HIV-1 isolates, and found that these two agents effectively suppressed their replication, although they did not compare the effects of CCR5 inhibitors or CXCR4 inhibitors plus other FDA-approved anti-HIV-1 agents (31). The mechanism of the potent synergism with AVC plus each of the two CXCR4 inhibitors observed in the present study is not clear at this time. In this regard, Singer et al. have demonstrated that CCR5, CXCR4, and CD4 are apposed predominantly on cellular microvilli and apparently form homogeneous microclusters in all cell types examined, including macrophages and T cells (34). Such a spatial distribution of the surface cellular molecules involved in HIV-1's cellular entry may be related to the observed antiviral synergism, possibly through the concurrent CCR5 inhibitor and CXCR4 inhibitor binding to target molecules, which might result in synergistic steric hindrance or conformational changes in such surface molecules, leading to the inhibition of HIV-1's gp120/gp41 binding to and/or fusion with the target cells.
In the present study, in order to assess the effects of the combination of AVC with other drugs, we developed a system, designated the Combo method, which provides (i) a flexible choice of interaction models, (ii) the use of nonparametric statistical methods to obtain P values for comparisons, and (iii) flexibility with respect to the experimental design (e.g., checkerboard and constant-ratio designs). It is of note that when AVC was combined with itself, there was an indication of synergism, as assessed by CIs greater than CI at an inhibitory effect of 75%. This result was thought to represent a limitation or error inherent to the variability of the cell-based assay data obtained and/or the median-effect method used. Indeed, as has been noted by others (21), a “combination effect” is often defined on the basis of the empirical CI values (e.g., <0.9 for synergy and >1.1 for antagonism), irrespective of the interassay variability, and no adjustments for multiple comparisons are generally made, producing an increased potential to overestimate the combination effects.
The variability of the cell-based assay data in determining the biological profiles of HIV-1, including its infectivity, replication competence, and cytopathic effect, stems from the very nature of the replication profiles of HIV-1 in culture. Unlike the bacterial multiplication profile, for which inhibition by antibiotics is arithmetically more predictable than is the case with HIV-1 replication, HIV-1 replication involves profuse but variable numbers of infectious progeny virions produced from a single infected cell. Indeed, the estimated number of progeny HIV-1 virions produced by a single HIV-1-exposed cell in our previous study ranged from 3.5 × 102 to 1.2 × 103 (14), while Layne et al. reported that such numbers ranged from 6 × 102 to 2.6 × 106 virions per cell (15). Moreover, the infectivity and replication competence of the HIV-1 inoculum can vary in the interassay as well as the intra-assay context. In fact, it is difficult to determine what portion of the virions used in a cell-based assay is infectious and replication competent. Layne et al. estimated the ratios of infectious to noninfectious virions using syncytium-forming units, which represents the infection of individual target cells by the number of cell-free virus counted (24) and which ranges from 4.1 × 10−4 to 3.6 × 10−7 (15). However, the number of virions produced in culture and their infectious potency also vary depending on the types of cells, particularly when target PHA-PBMCs are generated from different donors. Also, there should be variability in terms of the infectivity depending on the conditions of how and where the virus inoculum was generated and stored prior to the assay (16). In addition, if 100% viral infectivity suppression is not achieved in cell culture, a viral breakthrough tends to occur, since a continuous increase in the HIV-1 inoculum size occurs over the culture period and also contributes to the variability of the antiviral data. It is also true that the cell-based assays measure the cumulative effects of inhibitors over multiple cycles, which results in a substantial overestimation of synergy (12). It is noteworthy that the standard deviations of the EC50s of the three CCR5 inhibitors (AVC, SCH-C, and TAK779) were relatively large (Table 1). In this regard, we have previously reported that much greater variability in the EC50s for AVC compared to that for other classes of antiretroviral agents can be seen (18, 23). This variability most likely stems from that fact that the amounts of CCR5 receptors expressed on PBMCs differ substantially from one PBMC donor to another. Thus, evaluations of the antiviral effects of drug combinations require cautious interpretation of the data, including the use of statistical analyses to judge whether the differences between drug combinations are significant.
Using the Combo method described here, we compared the %synergymean values for AVC-AMD3100 with those for AVC-ENF, AVC-ZDV, AVC-NVP, and AVC-IDV (Fig. 5). Except in two instances, the effect of the combination of AVC with AMD3100 or TE14011 did not statistically exceed the effects of AVC plus other FDA-approved antiviral agents (Fig. 5) when the %synergymean values were examined. This observation that the synergism of AVC and a CXCR4 inhibitor failed to significantly exceed the effect of AVC in combination with the other antiviral agents tested could be due to the fact that the present study was not formally designed or powered for a direct comparison of the %synergymean values of AVC-AMD3100 and AVC-TE14011 against those of AVC plus FDA-approved antiretroviral drugs, with the result being that the number of experimental results evaluated for this comparison was quite small (n = 5). Larger experiments would likely be able to detect these differences as being significant. However, in our limited experiments, there was a tendency for the %synergymean values to be greater when AVC was combined with CXCR4 inhibitors than when AVC was combined with other compounds, even when this was not demonstrated statistically.
In the present study, cytotoxicity was virtually negligible at all concentrations and with all combinations examined. However, it is of note that although in the preclinical testing of AVC it was administered to monkeys at high doses over 9 months and no toxicity was observed, in phase IIb clinical trials, AVC caused grade 4 hepatotoxicity in 5 of 281 individuals receiving the drug and its development was abruptly terminated (25). Nevertheless, another CCR5 inhibitor, MVC, has been well tolerated, exerts significant antiviral effects, and has now been clinically used, suggesting that the hepatotoxicity of AVC is due to its chemical/structural properties and is not inherent to CCR5 inhibitors.
In conclusion, the present data demonstrate a tendency toward greater synergism when AVC is combined with either of the two CXCR4 inhibitors than when AVC is combined with other FDA-approved anti-HIV-1 agents, suggesting that the development of effective CXCR4 inhibitors may be important to increasing the efficacies of CCR5 inhibitors.
Acknowledgments
This work was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, and in part by a Grant-in-Aid for Scientific Research (Priority Areas) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Monbu-Kagakusho), a Grant for Promotion of AIDS Research from the Ministry of Health, Welfare, and Labor of Japan (Kosei-Rohdosho; grant H15-AIDS-001), and a Grant to the Cooperative Research Project on Clinical and Epidemiological Studies of Emerging and Reemerging Infectious Diseases (Renkei Jigyo, grant 78, Kumamoto University) of Monbu-Kagakusho.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou, T. C., and P. Talalay. 1981. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur. J. Biochem. 115:207-216. [DOI] [PubMed] [Google Scholar]
- 4.Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. [DOI] [PubMed] [Google Scholar]
- 5.Daar, E. S., K. L. Kesler, C. J. Petropoulos, W. Huang, M. Bates, A. E. Lail, E. P. Coakley, E. D. Gomperts, and S. M. Donfield. 2007. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin. Infect. Dis. 45:643-649. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq, E. 2002. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 1:13-25. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq, E., N. Yamamoto, R. Pauwels, J. Balzarini, M. Witvrouw, K. De Vreese, Z. Debyser, B. Rosenwirth, P. Peichl, R. Datema, et al. 1994. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob. Agents Chemother. 38:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demarest, J., T. Bonny, C. Vavro, C. Labranche, K. Kitrinos, C. Mcdanal, S. Sparks, S. Chavers, S. Castillo, D. Elrick, D. Mccarty, J. Whitcomb, W. Huang, C. Petropoulos, and S. Piscitell. 2004. HIV-1 co-receptor tropism in treatment naive and experienced subjects, abstr. H-1136. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 10.Dorr, P., M. Westby, S. Dobbs, P. Griffin, B. Irvine, M. Macartney, J. Mori, G. Rickett, C. Smith-Burchnell, C. Napier, R. Webster, D. Armour, D. Price, B. Stammen, A. Wood, and M. Perros. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fätkenheuer, G., A. L. Pozniak, M. A. Johnson, A. Plettenberg, S. Staszewski, A. I. Hoepelman, M. S. Saag, F. D. Goebel, J. K. Rockstroh, B. J. Dezube, T. M. Jenkins, C. Medhurst, J. F. Sullivan, C. Ridgway, S. Abel, I. T. James, M. Youle, and E. van der Ryst. 2005. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 11:1170-1172. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson, N. M., C. Fraser, and R. M. Anderson. 2001. Viral dynamics and anti-viral pharmacodynamics: rethinking in vitro measures of drug potency. Trends Pharmacol. Sci. 22:97-100. [DOI] [PubMed] [Google Scholar]
- 13.Gartner, S., P. Markovits, D. M. Markovitz, M. H. Kaplan, R. C. Gallo, and M. Popovic. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215-219. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama, S., D. T. Hoekzema, Y. Murakawa, E. Kojima, T. Shirasaka, D. J. Kempf, D. W. Norbeck, J. Erickson, and H. Mitsuya. 1994. A C2 symmetry-based HIV protease inhibitor, A77003, irreversibly inhibits infectivity of HIV-1 in vitro. AIDS Res. Hum. Retrovir. 10:735-743. [DOI] [PubMed] [Google Scholar]
- 15.Layne, S. P., M. J. Merges, M. Dembo, J. L. Spouge, S. R. Conley, J. P. Moore, J. L. Raina, H. Renz, H. R. Gelderblom, and P. L. Nara. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189:695-714. [DOI] [PubMed] [Google Scholar]
- 16.Looney, D. J., S. Hayashi, M. Nicklas, R. R. Redfield, S. Broder, F. Wong-Staal, and H. Mitsuya. 1990. Differences in the interaction of HIV-1 and HIV-2 with CD4. J. Acquir. Immune Defic. Syndr. 3:649-657. [PubMed] [Google Scholar]
- 17.Maeda, K., D. Das, H. Ogata-Aoki, H. Nakata, T. Miyakawa, Y. Tojo, R. Norman, Y. Takaoka, J. Ding, G. F. Arnold, E. Arnold, and H. Mitsuya. 2006. Structural and molecular interactions of CCR5 inhibitors with CCR5. J. Biol. Chem. 281:12688-12698. [DOI] [PubMed] [Google Scholar]
- 18.Maeda, K., H. Nakata, Y. Koh, T. Miyakawa, H. Ogata, Y. Takaoka, S. Shibayama, K. Sagawa, D. Fukushima, J. Moravek, Y. Koyanagi, and H. Mitsuya. 2004. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virol. 78:8654-8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda, K., K. Yoshimura, S. Shibayama, H. Habashita, H. Tada, K. Sagawa, T. Miyakawa, M. Aoki, D. Fukushima, and H. Mitsuya. 2001. Novel low molecular weight spirodiketopiperazine derivatives potently inhibit R5 HIV-1 infection through their antagonistic effects on CCR5. J. Biol. Chem. 276:35194-35200. [DOI] [PubMed] [Google Scholar]
- 20.Mosier, D. E., G. R. Picchio, R. J. Gulizia, R. Sabbe, P. Poignard, L. Picard, R. E. Offord, D. A. Thompson, and J. Wilken. 1999. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J. Virol. 73:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murga, J. D., M. Franti, D. C. Pevear, P. J. Maddon, and W. C. Olson. 2006. Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 50:3289-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakata, H., M. Amano, Y. Koh, E. Kodama, G. Yang, C. M. Bailey, S. Kohgo, H. Hayakawa, M. Matsuoka, K. S. Anderson, Y. C. Cheng, and H. Mitsuya. 2007. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob. Agents Chemother. 51:2701-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakata, H., K. Maeda, T. Miyakawa, S. Shibayama, M. Matsuo, Y. Takaoka, M. Ito, Y. Koyanagi, and H. Mitsuya. 2005. Potent anti-R5 human immunodeficiency virus type 1 effects of a CCR5 antagonist, AK602/ONO4128/GW873140, in a novel human peripheral blood mononuclear cell nonobese diabetic-SCID, interleukin-2 receptor gamma-chain-knocked-out AIDS mouse model. J. Virol. 79:2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nara, P. L., and P. J. Fischinger. 1988. Quantitative infectivity assay for HIV-1 and-2. Nature 332:469-470. [DOI] [PubMed] [Google Scholar]
- 25.Nichols, W. G., H. M. Steel, T. Bonny, K. Adkison, L. Curtis, J. Millard, K. Kabeya, and N. Clumeck. 2008. Hepatotoxicity observed in clinical trials of aplaviroc (GW873140). Antimicrob. Agents Chemother. 52:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizawa, R., T. Nishiyama, K. Hisaichi, N. Matsunaga, C. Minamoto, H. Habashita, Y. Takaoka, M. Toda, S. Shibayama, H. Tada, K. Sagawa, D. Fukushima, K. Maeda, and H. Mitsuya. 2007. Spirodiketopiperazine-based CCR5 antagonists: lead optimization from biologically active metabolite. Bioorg. Med. Chem. Lett. 17:727-731. [DOI] [PubMed] [Google Scholar]
- 27.Prichard, M. N., L. E. Prichard, W. A. Baguley, M. R. Nassiri, and C. Shipman, Jr. 1991. Three-dimensional analysis of the synergistic cytotoxicity of ganciclovir and zidovudine. Antimicrob. Agents Chemother. 35:1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prichard, M. N., L. E. Prichard, and C. Shipman, Jr. 1993. Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob. Agents Chemother. 37:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-205. [DOI] [PubMed] [Google Scholar]
- 30.Raport, C. J., J. Gosling, V. L. Schweickart, P. W. Gray, and I. F. Charo. 1996. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J. Biol. Chem. 271:17161-17166. [DOI] [PubMed] [Google Scholar]
- 31.Rusconi, S., S. La Seta Catamancio, P. Citterio, E. Bulgheroni, F. Croce, S. H. Herrmann, R. E. Offord, M. Galli, and M. S. Hirsch. 2000. Combination of CCR5 and CXCR4 inhibitors in therapy of human immunodeficiency virus type 1 infection: in vitro studies of mixed virus infections. J. Virol. 74:9328-9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saag, M., P. Ive, J. Heera, M. Tawadrous, E. DeJesus, N. Clumeck, D. Cooper, A. Horban, L. Mohapi, H. Mingrone, G. Reyes-Teran, S. Walmsley, F. Hackman, E. Ryst, and H. Mayer. 2007. A multicenter, randomized, double-blind, comparative trial of a novel CCR5 antagonist, maraviroc versus efavirenz, both in combination with Combivir (zidovudine [ZDV]/lamivudine [3TC]), for the treatment of antiretroviral naive patients infected with R5 HIV 1: week 48 results of the MERIT study. 4th Int. AIDS Soc. Conf. HIV Pathogenesis, Treatment, Prevention, abstr. WESS104.
- 33.Shirasaka, T., R. Yarchoan, M. C. O'Brien, R. N. Husson, B. D. Anderson, E. Kojima, T. Shimada, S. Broder, and H. Mitsuya. 1993. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc. Natl. Acad. Sci. USA 90:562-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer, I. I., S. Scott, D. W. Kawka, J. Chin, B. L. Daugherty, J. A. DeMartino, J. DiSalvo, S. L. Gould, J. E. Lineberger, L. Malkowitz, M. D. Miller, L. Mitnaul, S. J. Siciliano, M. J. Staruch, H. R. Williams, H. J. Zweerink, and M. S. Springer. 2001. CCR5, CXCR4, and CD4 are clustered and closely apposed on microvilli of human macrophages and T cells. J. Virol. 75:3779-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Liu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, J. R. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C. C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamamura, H., K. Hiramatsu, S. Kusano, S. Terakubo, N. Yamamoto, J. O. Trent, Z. Wang, S. C. Peiper, H. Nakashima, A. Otaka, and N. Fujii. 2003. Synthesis of potent CXCR4 inhibitors possessing low cytotoxicity and improved biostability based on T140 derivatives. Org. Biomol. Chem. 1:3656-3662. [DOI] [PubMed] [Google Scholar]
- 37.Tamamura, H., K. Hiramatsu, M. Mizumoto, S. Ueda, S. Kusano, S. Terakubo, M. Akamatsu, N. Yamamoto, J. O. Trent, Z. Wang, S. C. Peiper, H. Nakashima, A. Otaka, and N. Fujii. 2003. Enhancement of the T140-based pharmacophores leads to the development of more potent and bio-stable CXCR4 antagonists. Org. Biomol. Chem. 1:3663-3669. [DOI] [PubMed] [Google Scholar]
- 38.Tremblay, C. L., F. Giguel, Y. Guan, T. C. Chou, K. Takashima, and M. S. Hirsch. 2005. TAK-220, a novel small-molecule CCR5 antagonist, has favorable anti-human immunodeficiency virus interactions with other antiretrovirals in vitro. Antimicrob. Agents Chemother. 49:3483-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremblay, C. L., F. Giguel, C. Kollmann, Y. Guan, T. C. Chou, B. M. Baroudy, and M. S. Hirsch. 2002. Anti-human immunodeficiency virus interactions of SCH-C (SCH 351125), a CCR5 antagonist, with other antiretroviral agents in vitro. Antimicrob. Agents Chemother. 46:1336-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 41.Westby, M., M. Lewis, J. Whitcomb, M. Youle, A. L. Pozniak, I. T. James, T. M. Jenkins, M. Perros, and E. van der Ryst. 2006. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J. Virol. 80:4909-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]