Abstract
Clostridium difficile-associated diarrhea (CDAD) is caused by the toxins the organism produces when it overgrows in the colon as a consequence of antibiotic depletion of normal flora. Conventional antibiotic treatment of CDAD increases the likelihood of recurrent disease by again suppressing normal bacterial flora. Tolevamer, a novel toxin-binding polymer, was developed to ameliorate the disease without adversely affecting normal flora. In the current study, tolevamer was tested for its ability to neutralize clostridial toxins produced by the epidemic BI/027 strains, thereby preventing toxin-mediated tissue culture cell rounding. The titers of toxin-containing C. difficile culture supernatants were determined using confluent cell monolayers, and then the supernatants were used in assays containing dilutions of tolevamer to determine the lowest concentration of tolevamer that prevented ≥90% cytotoxicity. Tolevamer neutralized toxins in the supernatants of all C. difficile strains tested. Specific antibodies against the large clostridial toxins TcdA and TcdB also neutralized the cytopathic effect, suggesting that tolevamer is specifically neutralizing these toxins and that the binary toxin (whose genes are carried by the BI/027 strains) is not a significant source of cytopathology against tissue culture cells in vitro.
Clostridium difficile is a significant cause of nosocomial antibiotic-associated diarrhea, with the bacterium being found in 10% to 20% of cases of this disease (22). In several countries, including Canada, the United Kingdom, and the United States, the incidence of C. difficile-associated diarrhea (CDAD) is rising (2, 39, 52), and the emergence of hypertoxigenic, highly virulent BI/027 strains of C. difficile has likely contributed to the recent increase in infection rates and mortality (14, 33, 44, 52, 55). New therapies are urgently needed.
Typically, CDAD results following the acquisition and proliferation of C. difficile in the gut subsequent to the disruption of the normal protective enteric bacteria following antibiotic therapy. Presumably, this reduction of normal flora results in the loss of colonization resistance. The pathology resulting in CDAD is caused by two high-molecular-weight toxins, TcdA and TcdB, produced by the vegetative form of pathogenic strains of C. difficile (25, 34). In vivo, these toxins demonstrate different pathological profiles. TcdA is an enterotoxin and secretagogue that induces diarrhea. TcdB does not elicit a fluid response and has been described as a cytotoxin (31). However, strains producing TcdB, but with TcdA deleted, are still capable of causing clinical disease, demonstrating that the pathophysiology of this disease remains incompletely understood (54).
Currently, CDAD is managed with the antibiotics vancomycin and metronidazole, which are only effective against the vegetative form of C. difficile. However, these treatments have additional limitations, including incomplete response rates and reinfection/recurrence rates as high as 47% (36, 42). It has been hypothesized that high recurrence rates may result from further antibiotic-mediated disruption of the normal colonic flora, giving C. difficile the opportunity to recover with little competition from the normal flora (15, 16, 47). Thus, nonantibiotic approaches would appear to have promise for breaking this cycle of recurrence.
Tolevamer is the salt of a soluble, high-molecular-weight (>400 kDa) anionic polymer that noncovalently binds C. difficile TcdA and TcdB (5, 8, 27). Because the polymer is not antimicrobial, treatment should permit the restoration of normal gut flora and prevention of recurrence. Phase 2 clinical trials with tolevamer, while not powered to statistically demonstrate reduced recurrence, showed a trend in this direction (7% recurrence among tolevamer-treated patients compared with 19% among those treated with vancomycin) (30). Although tolevamer did not meet its primary efficacy end point in phase 3 trials (unpublished data), patients treated with tolevamer continued to show a reduction in recurrence rate: 3% for tolevamer compared with 23% for vancomycin and 27% for metronidazole (29).
In 2005, studies published by Loo et al. (28) and McDonald et al. (33) described C. difficile outbreaks in Canada and the United States, respectively. In both studies, greater than 50% of the C. difficile isolates were characterized as being restriction endonuclease analysis group BI, pulsed-field gel electrophoresis type NAP1, toxinotype III, and PCR ribotype 027 (26, 48). These strains appeared to be associated with more severe disease (3, 4), to contain genes for the C. difficile binary toxin, and to overexpress both TcdA and TcdB when cultured in vitro (55) due to a frameshift at position 117 of the tcdC gene in the pathogenicity locus (11, 32). The BI/027 strains have also been associated with increased fluoroquinolone resistance (23). In the studies presented here, we sought to determine whether tolevamer could neutralize toxins from these more virulent strains.
MATERIALS AND METHODS
Clostridium difficile strains.
Isolates used in these studies were obtained from the American Type Culture Collection (ATCC) or were kindly provided by investigators as indicated in Table 1.
TABLE 1.
Sources and providers of the isolates from this study
| Isolate | Source and/or characteristic(s) | Provider |
|---|---|---|
| VL00013 | Montreal; BI/027 | T. Louie, University of Calgary, Canada |
| 2004013 | Maine; BI/027 | Angela Thompson, CDC |
| VA-21 | Georgia; BI/027 | Angela Thompson, CDC |
| 2004101 | Maine; BI/027 | Angela Thompson, CDC |
| 2004163 | Pennsylvania; BI/027 | Angela Thompson, CDC |
| 678-147 | Great Britain | M. Wilcox, University of Leeds, Great Britain |
| 43594 | ATCC | ATCC |
| 8864 | Clinical isolate; TcdA− TcdB+ | D. Willis, Meridian Diagnostics |
| DiPersio | Clinical isolate; TcdA− TcdB+ | D. Willis, Meridian Diagnostics |
| 17857 | ATCC | ATCC |
| 43596 | ATCC; serogroup C | ATCC |
| 17858 | ATCC | ATCC |
| 9689 | ATCC; type strain | ATCC |
| 43598 | ATCC | ATCC |
| 43599 | ATCC | ATCC |
| 43600 | ATCC | ATCC |
| CD196 | Clinical isolate; high binary toxin producer (49) | M. Popoff, Institut Pasteur, France |
Culture supernatant preparation.
C. difficile isolates were streaked on prereduced brucella blood agar plates (Anaerobe Systems) and were incubated for 24 h at 35°C in canisters containing an Anaeropac (Mitsubishi Gas Chemical Co.) to produce an anaerobic atmosphere. A single colony was inoculated into 5 ml of prereduced brain heart infusion medium (Anaerobe Systems) and was incubated for 48 h at 35°C. One and a half milliliters of culture supernatant was centrifuged (5 min at 5,000 × g) to pellet bacterial cells, and the C. difficile culture supernatant was filter sterilized through a 0.22-μm nylon filter (Pall Acrodisk) prior to storage at 4°C until use.
Tissue culture.
Four different cell types were analyzed for sensitivity to clostridial toxins. Vero cells (African green monkey kidney cells; ATCC CCL-81) were grown in minimal essential medium (MEM) containing 10% fetal bovine serum (FBS). Caco-2 cells (colorectal adenocarcinoma cells; ATCC HTB-37) were cultured in MEM with 1% nonessential amino acids plus 20% FBS. CCD-18Co cells (human colon fibroblasts; ATCC CRL-1459) were cultured in Eagle's MEM plus 10% FBS, and T84 cells (human colorectal carcinoma epithelial cells; ATCC CCL-248) were cultured in a 1:1 mixture of Dulbecco's MEM-Ham's F-12 medium plus 5% FBS. To prepare confluent monolayers, Vero cells were dispensed into 96-well plates at 4 × 104 cells/well, Caco-2 cells at 2 × 104 cells/well, CCD-18Co cells at 3 × 104 cells/well, and T84 cells at 5 × 104 cells/well. Plates were incubated for 24 h at 37°C in 5% CO2 to reach confluence before the experiments were set up.
Toxin titration.
Toxin titer was determined by performing serial twofold dilutions of C. difficile culture supernatants in tissue culture medium, applying these dilutions to confluent cell monolayers, and incubating them for 24 h at 37°C in 5% CO2. The cytopathic effect was then determined by microscopic examination, as previously described (51). All titrations were performed in triplicate, and the average was determined for use in subsequent neutralization studies. For neutralization studies, the concentration of C. difficile culture supernatant selected corresponded to twofold more than the average minimum dilution required to achieve >90% cell rounding. Antibody neutralization studies utilized four times the minimum cytopathic concentration to account for the possible variation in the molar ratios of toxins produced by different strains (23). Purified C. difficile toxin A and toxin B (Genzyme Diagnostics) were included as controls.
Tolevamer-mediated toxin neutralization.
The ability of tolevamer (GT267-004; Genzyme Corp, Haverhill, United Kingdom) to neutralize clostridial toxins found in culture supernatants was determined as previously described (27). Briefly, tolevamer was serially diluted across a microtiter plate in 1/10-fold dilutions in tissue culture medium plus 10% FBS. Appropriately diluted purified toxin or C. difficile culture supernatant was added, and the mixture was immediately transferred to wells containing confluent Vero cell monolayers. (Previous work [unpublished] has shown no differences in toxin neutralization when the mixture was incubated separately for 1 h before being added to the cells versus when it was added immediately.) Plates were incubated for 24 h at 37°C, and the cytopathic effect was analyzed by microscopic examination at ×200 magnification. The cutoff point for tolevamer neutralization was defined as that concentration of tolevamer that prevented ≥90% cell rounding (i.e., that allowed ≤10% rounded cells/well, with >90% protected). Dilution series were performed in triplicate for each culture supernatant analyzed, and experiments were repeated three times.
Antibody-mediated toxin neutralization.
Toxin-specific antibodies (goat anti-toxin A polyclonal and rabbit anti-toxin B polyclonal [kindly provided by Jeremy Schonhorn, Genzyme Diagnostics]) were tested for their capacity to neutralize the cytopathic effects of purified TcdA and TcdB and C. difficile culture supernatants applied to T84 cells. T84 cells were used for the antibody neutralization studies because their greatly reduced sensitivity to TcdB required far less anti-TcdB antibody to demonstrate neutralization. Vero cells were used for tolevamer neutralization because the C. difficile cytotoxicity assay utilizing these cells represents the gold standard (38). Assays were performed as described above for tolevamer neutralization, except that antibodies were diluted twofold across the plate.
RESULTS
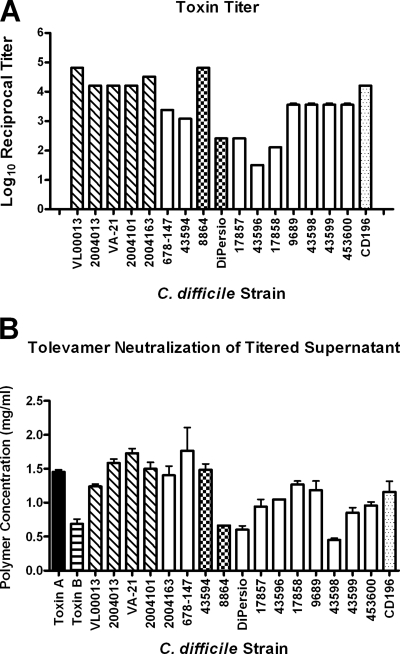
Isolates of C. difficile were grown in broth culture, and the titer of the filter-sterilized C. difficile culture supernatant was determined in the cell-rounding assay using Vero cells, which has long been the gold standard for C. difficile toxin detection (38). The results (Fig. 1A) showed a range in log10 reciprocal titers (the lowest dilution which caused 100% cell rounding) from 1.51 (strain 45396) to 4.82 (strains VL00013 and 8864). Most strains had log10 reciprocal titers greater than 3.0. The log10 reciprocal of the geometric mean of the five BI/027 isolates (Fig. 1) collectively was 4.39, which is approximately 15-fold higher than the geometric mean of the other strains tested (log10 reciprocal titer, 3.20). The values shown in Fig. 1 represent the averages of the results from three experiments.
FIG. 1.
(A) Toxin titers of C. difficile culture supernatants measured by cytotoxicity against Vero cells. Log10 reciprocal titer, the reciprocal of the log of the lowest dilution of C. difficile culture supernatant required for >90% cell rounding. (B) Concentration of tolevamer required to neutralize toxins. Diagonally hatched bars, BI/027 strains; white bars, other C. difficile strains, primarily from ATCC; checkered bars, strains expressing TcdB only (no TcdA); dotted bars, strains producing a large amount of binary toxins; black bar, purified TcdA; horizontally hatched bar, purified TcdB. Error bars are the standard deviations. All C. difficile culture supernatants were tested at least three times, except for strains 8864 and 43596, which were tested only twice.
Tolevamer neutralizes cytotoxic activity in all C. difficile strains tested.
We evaluated the capacity of tolevamer to neutralize toxins found in the C. difficile culture supernatants. For these studies, C. difficile culture supernatants were applied to Vero cells at twice the toxin titer, and then tolevamer was diluted across the plate. The results (Fig. 1B) represent the lowest concentration of tolevamer that prevented ≥90% cell rounding. The amount of tolevamer required for neutralization varied little for the strains tested, ranging from a low of 0.45 mg/ml (strain 43598) to a high of 1.76 mg/ml (strain 678-147). For reference, 0.8 ng of purified TcdA required 1.45 mg/ml of tolevamer. The neutralization of 0.0125 ng/ml of purified TcdB required 0.625 mg/ml of tolevamer.
Tissue culture cells differ in sensitivity to clostridial toxins.
Studies were done to determine the sensitivity of different cell lines to TcdA and TcdB. Purified toxins were diluted across 96-well plates containing confluent monolayers. The plates were incubated for 24 h and then examined microscopically. Table 2 shows the lowest concentration of purified toxin that still produced 100% cell rounding, averaged from the results of three experiments. Vero cells, the gold standard, are modestly sensitive to TcdA but exquisitely sensitive to TcdB, whereas T84 cells, from a human intestinal epithelial cell line, display relatively similar sensitivities to TcdA and TcdB. Presumably, this is because of a different distribution of toxin-binding receptors on the cell surface (10).
TABLE 2.
Sensitivity of different cell lines to C. difficile toxins TcdA and TcdBa
| Cell line | Concn of toxin (ng/ml) required to cause 100% cell rounding
|
Fold difference | |
|---|---|---|---|
| TcdA | TcdB | ||
| Vero | 4 | 0.0125 | 320 |
| Caco-2 | 5.75 | 0.22 | 26 |
| CCD-18Co | 248 | 0.00098 | 253,725 |
| T84 | 2.44 | 4.88 | 2 |
Confluent monolayers of the indicated cell lines were incubated for 24 h in serial dilutions of purified TcdA or TcdB. The cytopathic effects were determined by microscopic examination. The fold difference is the ratio of the concentration of TcdA divided by the concentration of TcdB (concentrations for 100% cell rounding).
Antibodies to TcdA and TcdB neutralize the cytopathic effects of purified clostridial toxins and also the toxins found in C. difficile culture supernatants.
To more precisely identify the toxins in C. difficile culture supernatants that are neutralized by tolevamer (Fig. 1B), we performed toxin neutralization studies using antibodies directed against purified TcdA and TcdB. Preliminary studies were done to determine the minimum amount of antibody required to neutralize the cytopathic effects of purified toxins. Purified toxin at four times the minimum required for 100% cell rounding was mixed with dilutions of antibody and then incubated for 24 h with confluent monolayers before microscopic examination. A total of 4.2 ng anti-TcdA was required to neutralize each ng of TcdA, whereas 300 ng of anti-TcdB was required to neutralize each ng of TcdB. Because of this difference, we chose to use T84 cells for the antibody neutralization studies, since the relatively small (twofold) difference in sensitivity to TcdA versus TcdB meant less antibody would be required for studying the neutralization of both toxins in BI/027 C. difficile culture supernatants.
For neutralizing toxins in C. difficile culture supernatants, we used concentrations of antibody four times higher than the minimum required for the neutralization of purified toxin to account for the variability in the molar ratios of TcdA and TcdB present (21). Confluent T84 monolayers were incubated for 24 h with titers of C. difficile culture supernatants from the five BI/027 strains either without antibody or with anti-TcdA alone, anti-TcdB alone, anti-TcdA plus anti-TcdB, or tolevamer and then were examined microscopically. Representative photomicrographs are shown in Fig. 2. In the absence of toxin, either alone or in the presence of anti-TcdA or anti-TcdB, cells in confluent monolayers remained adherent and nonrefractile (Fig. 2, top panels). The middle three panels of Fig. 2 show the effect of C. difficile culture supernatant. In the presence of either anti-TcdA or anti-TcdB only, the cytopathic effects of the toxins were not affected and monolayers of T84 cells became refractile and patchy in appearance. Only when both antibodies were present was the cytopathic effect of the C. difficile culture supernatant neutralized (Fig. 2, middle right panel), indicating that both TcdA and TcdB were present in the C. difficile culture supernatant. Further, this showed that no other toxins (such as binary toxin) were present in concentrations high enough to elicit cytopathic effects at the supernatant concentrations tested. The bottom panels in Fig. 2 illustrate the cytopathic effects of purified TcdA and TcdB (left and middle) and the neutralizing effect of tolevamer against the supernatant (right). Similar neutralizing results were obtained using tolevamer against purified TcdA or TcdB (data not shown).
FIG. 2.
Representative photomicrographs (viewed at ×200 magnification) of T84 cells in the presence or absence of purified large clostridial toxins TcdA and TcdB, antibodies directed against TcdA (Anti-A) and TcdB (Anti-B), tolevamer, or C. difficile culture supernatant (Sup). Studies were repeated three times. Top panels, controls showing the absence or presence of antibodies to TcdA or TcdB alone in the absence of toxin; middle panels, C. difficile culture supernatant plus antibodies alone or in combination; bottom panels, purified TcdA and TcdB alone and C. difficile culture supernatant plus tolevamer. These panels illustrate the results of using C. difficile culture supernatant from strain VL00013; the results with other BI/027 supernatants were similar. The results from using tolevamer to neutralize purified TcdA and TcdB were similar to those for the C. difficile culture supernatant (data not shown).
DISCUSSION
There is little doubt that both the incidence and severity of CDAD are increasing (9, 23, 28, 37, 43). The reasons for this are not entirely clear but probably reflect both patient-related issues, such as changing demographics and underlying disease (41), and pathogen-related factors, such as the spread of the BI/027 strains of C. difficile (3, 4, 14, 33, 44, 52). Warny and coworkers reported the in vitro production of TcdA and TcdB to be 16- and 23-fold greater, respectively, than that of reference strains of toxinotype O (55). Titers of the BI/027 C. difficile culture supernatants analyzed here (Fig. 1) were ∼15-fold greater than the average of the other strains examined, which is similar to the values reported by Warny. In addition to the BI/027 strains associated with recent outbreaks, our panel included two strains (8864 and Dipersio) which are TcdA− and TcdB+ and one strain (CD196) that produces high amounts of binary toxin.
Tolevamer neutralized toxins in the titered C. difficile culture supernatants with efficacies similar for all strains, though the absolute amount of tolevamer required appeared to be slightly higher for the BI/027 strains (Fig. 1B). However, the limit of discrimination in dose-down experiments depends upon the dilution factor—in this case, 2. Therefore, in this study, the absolute values can vary by a factor of 2, suggesting that the amount of tolevamer required for neutralization was functionally equivalent for all the strains examined, including the BI/027 strains.
Assessing the clinical relevance of the observed in vitro increase in toxin production among BI/027 strains is difficult. C. difficile toxin production in vitro varies widely, depending upon the composition of the medium (13, 21, 24), the strain, and the presence of some antibiotics with sub-MIC levels (including metronidazole) (12, 40). Few studies have examined toxin levels in vivo, in part because assessing toxin titers in fecal samples is not trivial; however, some studies have attempted to correlate toxin production in vitro and in vivo with disease severity (1, 6, 17, 35, 56). While there appears to be a trend toward a correlation between high toxin production in vivo and disease severity, the largest study to date comparing in vitro and in vivo toxin production demonstrated no correlation between the amount of toxin recovered in stool samples and the capacity of the recovered clinical isolates to produce toxin in vitro (1). This lack of correlation is not limited to clinical observations, since studies with hamster models have also failed to yield a correlation between virulence and the amount of toxin produced in in vitro cultures (6).
The reasons for this lack of concordance are not clear. C. difficile isolates recovered from stool samples by microbial culture may not represent the prevalent toxin-producing phenotype strains in vivo (53). Alternatively, the lack of concordance could reflect the complexity and variability of the factors affecting bacterial growth and toxin production in the colon (6, 35, 58, 60). However, until definitive clinical studies are performed comparing disease severity with levels of fecal toxin production by BI/027 strains of C. difficile, assessing the clinical consequence of altered in vitro TcdA and TcdB production by these strains remains uncertain.
In addition to carrying TcdA and TcdB, the BI/027 strains have also been shown to carry genes for the binary toxins CdtA and CdtB (33). Together, these introduce an actin-specific ADP-ribosyltransferase (related to the iota toxins produced by C. perfringens) into the cytosol (7, 46). Unlike the large clostridial toxins TcdA and TcdB, the role of binary toxins in disease pathology is not clear. In vitro, cytotoxicity due to binary toxins cannot be detected unless supernatants are concentrated ∼40-fold by ammonium sulfate precipitation (45). Perhaps because of this requirement, the great majority of studies identify the presence of the binary toxin genes but do not assay either cytotoxicity or enzymatic activity (19, 20, 28, 33, 50). The few studies that have done so identified the strain CD196 as a high toxin producer (compared with the production of binary toxins by other strains) (49). Notably, the C. difficile culture supernatant cytopathic activity of this strain was neutralized by tolevamer in the present study (Fig. 1B); however, this was most likely due to TcdA and TcdB rather than binary toxin.
In vivo, purified binary toxin produces vigorous secretion in rabbit ileal loops (18). However, while TcdA− TcdB− Cdt+ C. difficile strains colonize hamsters following clindamycin treatment, these strains do not cause disease or altered pathology (18). Some clinical studies have suggested that C. difficile isolates bearing CdtA and CdtB genes appear to be associated with increased disease severity (3), but other studies have noted that at least some BI/027 isolates containing these genes were not associated with more severe clinical disease (26). Taking these results together, it has been hypothesized that while binary toxins may exacerbate disease caused by TcdA and TcdB, it is not clear that binary toxins are capable of inducing disease on their own (26). Studies examining whether tolevamer neutralizes binary toxins have been initiated.
Two large, controlled international phase 3 clinical trials evaluating the efficacy of tolevamer compared to that of vancomycin and metronidazole in the treatment of CDAD were completed in 2007. The primary efficacy variable was clinical success, defined as the presence of explicit data indicating diarrhea resolution and the absence of severe abdominal discomfort due to CDAD on day 10. To date, the results of the first study have been presented, and tolevamer was shown to be inferior to both vancomycin and metronidazole for the resolution of CDAD, though it was associated with a lower recurrence rate than antibiotic therapy (29). The reasons for the disconnect between tolevamer's efficacy in both in vitro and in vivo models and the disappointing results seen in the phase 3 clinical program are unknown, but possible explanations include inadequate polymer concentration or toxin-binding affinity at relevant anatomic sites, either through interfering substances in the gut lumen occupying binding sites, impaired access to the mucosal surface, or insufficient clinical dosing. In the phase 2 study (30), the 6 g/day dose of tolevamer used was calculated (8) to be sufficient to neutralize the ∼1 μg/ml of toxin estimated by McFarland et al. (35). Due to technical constraints, and the limited correlation between toxin concentration and manifestations of clinical disease, neither toxin nor tolevamer fecal concentrations were measured in the phase 3 studies. Therefore, whether tolevamer was present at a high enough concentration in the phase 3 studies cannot be ascertained.
Acknowledgments
We thank David Davidson and John Leonard for helpful discussions throughout this work.
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Akerlund, T., B. Svenungsson, A. Lagergren, and L. G. Burman. 2006. Correlation of disease severity with fecal toxin levels in patients with Clostridium difficile-associated diarrhea and distribution of PCR ribotypes and toxin yields in vitro of corresponding isolates. J. Clin. Microbiol. 44:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald, L. K., S. N. Banerjee, and W. R. Jarvis. 2004. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987-2001. J. Infect. Dis. 189:1585-1588. [DOI] [PubMed] [Google Scholar]
- 3.Barbut, F., D. Decre, V. Lalande, B. Burghoffer, L. Noussair, A. Gigandon, F. Espinasse, L. Raskine, J. Robert, A. Mangeol, C. Branger, and J. C. Petit. 2005. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J. Med. Microbiol. 54:181-185. [DOI] [PubMed] [Google Scholar]
- 4.Barbut, F., B. Gariazzo, L. Bonne, V. Lalande, B. Burghoffer, R. Luiuz, and J. C. Petit. 2007. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect. Control Hosp. Epidemiol. 28:131-139. [DOI] [PubMed] [Google Scholar]
- 5.Barker, R. H., Jr., R. Dagher, D. M. Davidson, and J. K. Marquis. 2006. Tolevamer, a novel toxin-binding polymer: overview of preclinical pharmacology and physiochemical properties. Aliment. Pharmacol. Ther. 24:1525-1534. [DOI] [PubMed] [Google Scholar]
- 6.Borriello, S. P., J. M. Ketley, T. J. Mitchell, F. E. Barclay, A. R. Welch, A. B. Price, and J. Stephen. 1987. Clostridium difficile—a spectrum of virulence and analysis of putative virulence determinants in the hamster model of antibiotic-associated colitis. J. Med. Microbiol. 24:53-64. [DOI] [PubMed] [Google Scholar]
- 7.Braun, M., C. Herholz, R. Straub, B. Choisat, J. Frey, J. Nicolet, and P. Kuhnert. 2000. Detection of the ADP-ribosyltransferase toxin gene (cdtA) and its activity in Clostridium difficile isolates from Equidae. FEMS Microbiol. Lett. 184:29-33. [DOI] [PubMed] [Google Scholar]
- 8.Braunlin, W., Q. Xu, P. Hook, R. Fitzpatrick, J. D. Klinger, R. Burrier, and C. B. Kurtz. 2004. Toxin binding of tolevamer, a polyanionic drug that protects against antibiotic-associated diarrhea. Biophys. J. 87:534-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2005. Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. MMWR Morb. Mortal. Wkly. Rep. 54:1201-1205. [PubMed] [Google Scholar]
- 10.Chaves-Olarte, E., M. Weidmann, C. Eichel-Streiber, and M. Thelestam. 1997. Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J. Clin. Investig. 100:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curry, S. R., J. W. Marsh, C. A. Muto, M. M. O'Leary, A. W. Pasculle, and L. H. Harrison. 2007. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J. Clin. Microbiol. 45:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond, L. J., D. G. Smith, and I. R. Poxton. 2003. Effects of sub-MIC concentrations of antibiotics on growth of and toxin production by Clostridium difficile. J. Med. Microbiol. 52:1033-1038. [DOI] [PubMed] [Google Scholar]
- 13.Dupuy, B., and A. L. Sonenshein. 1998. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 27:107-120. [DOI] [PubMed] [Google Scholar]
- 14.Eggertson, L., and B. Sibbald. 2004. Hospitals battling outbreaks of C. difficile. CMAJ 171:19-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fekety, R., L. V. McFarland, C. M. Surawicz, R. N. Greenberg, G. W. Elmer, and M. E. Mulligan. 1997. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin. Infect. Dis. 24:324-333. [DOI] [PubMed] [Google Scholar]
- 16.Fekety, R., and A. B. Shah. 1993. Diagnosis and treatment of Clostridium difficile colitis. JAMA 269:71-75. [PubMed] [Google Scholar]
- 17.George, W. L., R. D. Rolfe, and S. M. Finegold. 1982. Clostridium difficile and its cytotoxin in feces of patients with antimicrobial agent-associated diarrhea and miscellaneous conditions. J. Clin. Microbiol. 15:1049-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geric, B., R. J. Carman, M. Rupnik, C. W. Genheimer, S. P. Sambol, D. M. Lyerly, D. N. Gerding, and S. Johnson. 2006. Binary toxin-producing, large clostridial toxin-negative Clostridium difficile strains are enterotoxic but do not cause disease in hamsters. J. Infect. Dis. 193:1143-1150. [DOI] [PubMed] [Google Scholar]
- 19.Geric, B., M. Rupnik, D. N. Gerding, M. Grabnar, and S. Johnson. 2004. Distribution of Clostridium difficile variant toxinotypes and strains with binary toxin genes among clinical isolates in an American hospital. J. Med. Microbiol. 53:887-894. [DOI] [PubMed] [Google Scholar]
- 20.Gonçalves, C., D. Decré, F. Barbut, B. Burghoffer, and J.-C. Petit. 2004. Prevalence and characterization of a binary toxin (actin-specific ADP-ribosyltransferase) from Clostridium difficile. J. Clin. Microbiol. 42:1933-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haslam, S. C., J. M. Ketley, T. J. Mitchell, J. Stephen, D. W. Burdon, and D. C. A. Candy. 1986. Growth of Clostridium difficile and production of toxins A and B in complex and defined media. J. Med. Microbiol. 21:293-297. [DOI] [PubMed] [Google Scholar]
- 22.Hogenauer, C., H. F. Hammer, G. J. Krejs, and E. C. Reisinger. 1998. Mechanisms and management of antibiotic-associated diarrhea. Clin. Infect. Dis. 27:702-710. [DOI] [PubMed] [Google Scholar]
- 23.Hookman, P., and J. S. Barkin. 2007. Review: Clostridium difficile-associated disorders/diarrhea and Clostridium difficile colitis: the emergence of a more virulent era. Dig. Dis. Sci. 52:1071-1075. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson, S., A. Lindberg, E. Norin, L. G. Burman, and T. Akerlund. 2000. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect. Immun. 68:5881-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, C. P., C. Pothoulakis, and J. T. LaMont. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330:257-262. [DOI] [PubMed] [Google Scholar]
- 26.Kuijper, E. J., R. J. van den Berg, S. Debast, C. E. Visser, D. Veenendaal, A. Troelstra, T. van der Kooi, S. van den Hof, and D. W. Notermans. 2006. Clostridium difficile ribotype 027, toxinotype III, the Netherlands. Emerg. Infect. Dis. 12:827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurtz, C. B., E. P. Cannon, A. Brezzani, M. Pitruzzello, C. Dinardo, E. Rinard, D. W. K. Acheson, R. Fitzpatrick, P. Kelly, K. Shackett, A. T. Papoulis, P. J. Goddard, R. H. Barker, Jr., G. P. Palace, and J. D. Klinger. 2001. GT160-246, a toxin binding polymer for treatment of Clostridium difficile colitis. Antimicrob. Agents Chemother. 45:2340-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 29.Louie, T. J., M. Gerson, S. Johnson, L. Poirier, K. Weiss, J. Peppe, J. Donovan, and D. Davidson. 2007. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhea (CDAD), abstr. 425a. Abstr. 47th. Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL.
- 30.Louie, T. J., J. Peppe, C. K. Watt, D. Johnson, R. Mohammed, G. Dow, K. Weiss, S. Simon, J. F. John, Jr., G. Garber, S. Chasan-Taber, and D. M. Davidson. 2006. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 43:411-420. [DOI] [PubMed] [Google Scholar]
- 31.Lyerly, D. M., D. E. Lockwood, S. H. Richardson, and T. D. Wilkins. 1982. Biological activities of toxins A and B of Clostridium difficile. Infect. Immun. 35:1147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matamouros, S., P. England, and B. Dupuy. 2007. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol. Microbiol. 64:1274-1288. [DOI] [PubMed] [Google Scholar]
- 33.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 34.McFarland, L. V. 2000. Normal flora: diversity and functions. Microb. Ecol. Health Dis. 12:193-207. [Google Scholar]
- 35.McFarland, L. V., G. W. Elmer, W. E. Stamm, and M. E. Mulligan. 1991. Correlation of immunoblot type, enterotoxin production, and cytotoxin production with clinical manifestations of Clostridium difficile infection in a cohort of hospitalized patients. Infect. Immun. 59:2456-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musher, D. M., S. Aslam, N. Logan, S. Nallacheru, I. Bhaila, F. Borchert, and R. J. Hamill. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. 40:1586-1590. [DOI] [PubMed] [Google Scholar]
- 37.Muto, C. A., M. Pokrywka, K. Shutt, A. B. Mendelsohn, K. Nouri, K. Posey, T. Roberts, K. Croyle, S. Krystofiak, S. Patel-Brown, A. W. Pasculle, D. L. Paterson, M. Saul, and L. H. Harrison. 2005. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect. Control Hosp. Epidemiol. 26:273-280. [DOI] [PubMed] [Google Scholar]
- 38.Nachamkin, I., L. Lotz-Nolan, and D. Skalina. 1986. Evaluation of a commercial cytotoxicity assay for detection of Clostridium difficile toxin. J. Clin. Microbiol. 23:954-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Clostridium difficile Standards Group. 2004. National Clostridium difficile Standards Group: report to the Department of Health. J. Hosp. Infect. 56(Suppl. 1):1-38. [DOI] [PubMed] [Google Scholar]
- 40.Onderdonk, A. B., B. R. Lowe, and J. G. Bartlett. 1979. Effect of environmental stress on Clostridium difficile toxin levels during continuous cultivation. Appl. Environ. Microbiol. 38:637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peled, N., S. Pitlik, Z. Samra, A. Kazakov, Y. Bloch, and J. Bishara. 2007. Predicting Clostridium difficile toxin in hospitalized patients with antibiotic-associated diarrhea. Infect. Control Hosp. Epidemiol. 28:377-381. [DOI] [PubMed] [Google Scholar]
- 42.Pepin, J., M. E. Alary, L. Valiquette, E. Raiche, J. Ruel, K. Fulop, D. Godin, and C. Bourassa. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 40:1591-1597. [DOI] [PubMed] [Google Scholar]
- 43.Pepin, J., L. Valiquette, M. E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pepin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 171:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pepin, J., L. Valiquette, and B. Cossette. 2005. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 173:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perelle, S., M. Gibert, P. Bourlioux, G. Corthier, and M. R. Popoff. 1997. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect. Immun. 65:1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popoff, M. R., E. J. Rubin, D. M. Gill, and P. Boquet. 1988. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 56:2299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pothoulakis, C., and J. T. LaMont. 1993. Clostridium difficile colitis and diarrhea. Gastroenterol. Clin. N. Am. 22:623-637. [PubMed] [Google Scholar]
- 48.Rupnik, M., V. Avesani, M. Janc, C. von Eichel-Streiber, and M. Delmee. 1998. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 36:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stubbs, S., M. Rupnik, M. Gibert, J. Brazier, B. Duerden, and M. Popoff. 2000. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 186:307-312. [DOI] [PubMed] [Google Scholar]
- 50.Terhes, G., E. Urban, J. Soki, K. A. Hamid, and E. Nagy. 2004. Community-acquired Clostridium difficile diarrhea caused by binary toxin, toxin A, and toxin B gene-positive isolates in Hungary. J. Clin. Microbiol. 42:4316-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres, J., M. Camorlinga-Ponce, and O. Munoz. 1992. Sensitivity in culture of epithelial cells from rhesus monkey kidney and human colon carcinoma to toxins A and B from Clostridium difficile. Toxicon 30:419-426. [DOI] [PubMed] [Google Scholar]
- 52.Valiquette, L., D. E. Low, J. Pepin, and A. McGeer. 2004. Clostridium difficile infection in hospitals: a brewing storm. CMAJ 171:27-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Berg, R. J., H. A. Ameen, T. Furusawa, E. C. Claas, E. R. van der Vorm, and E. J. Kuijper. 2005. Coexistence of multiple PCR-ribotype strains of Clostridium difficile in faecal samples limits epidemiological studies. J. Med. Microbiol. 54:173-179. [DOI] [PubMed] [Google Scholar]
- 54.Voth, D. E., and J. D. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warny, M., J. Pepin, A. Fang, G. E. Killgore, J. Thompson, J. Brazier, E. Frost, and L. C. McDonald. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079-1084. [DOI] [PubMed] [Google Scholar]
- 56.Wren, B. W., S. R. Heard, and S. Tabaqchili. 1987. Association between production of toxins A and B and types of Clostridium difficile. J. Clin. Pathol. 40:1397-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]