Abstract
Cefditoren is a broad-spectrum, oral cephalosporin that is highly active against clinically relevant respiratory tract pathogens, including multidrug-resistant Streptococcus pneumoniae. This study described its pharmacodynamic profile in plasma and epithelial lining fluid (ELF). Plasma and ELF pharmacokinetic data were obtained from 24 patients under fasting conditions. Cefditoren and urea concentrations were determined in plasma and bronchoalveolar lavage fluid by liquid chromatography-tandem mass spectrometry. Concentration-time profiles in plasma and ELF were modeled using a model with three disposition compartments and first-order absorption, elimination, and transfer. Pharmacokinetic parameters were identified in a population pharmacokinetic analysis (big nonparametric adaptive grid with adaptive γ). Monte Carlo simulation (9,999 subjects) was performed with the ADAPT II program to estimate the probability of target attainment at which the free-cefditoren plasma concentrations (88%) protein binding and total ELF concentrations exceeded the MIC for 33% of the dosing interval for 400 mg cefditoren given orally every 12 h. After the Bayesian step, the overall fits of the model to the data were good, and plots of predicted versus observed concentrations for plasma and ELF showed slopes and intercepts very close to the ideal values of 1.0 and 0.0, respectively. In the plasma probability of target attainment analysis, the probability of achieving a time for which free, or unbound, plasma concentration exceeds the MIC of the organism for 33% of the dosing interval was <80% for a MIC of >0.06 mg/liter. Similar to plasma, the probability of achieving a time above the MIC of 33% was <80% for MIC of >0.06 mg/liter in ELF. Cefditoren was found to have a low probability of achieving a bacteriostatic effect against MICs of >0.06 mg/liter, which includes most S. pneumoniae isolates with intermediate susceptibility to penicillin, when given in the fasting state in both plasma and ELF.
Lower respiratory tract infections remain the most common infections observed in the community and among hospitalized patients (1, 5, 6, 11, 32, 39, 41). In the community setting, lower respiratory tract infections account for the largest number of clinic visits and are the most frequent cause of antibiotic prescriptions (5, 6, 39). During the past 2 decades, the emergence of antibiotic resistance, particularly multidrug-resistant Streptococcus pneumoniae, has resulted in new challenges in the treatment of lower respiratory tract infections (1, 5, 8, 10, 22, 23, 44, 45, 48, 52). To ensure the highest probability of a favorable clinical response, empirical therapy for lower respiratory tract infections requires the prompt delivery of antibiotics with activity against the most likely pathogens, including multidrug-resistant S. pneumoniae (5, 31).
A recent addition to the armamentarium against lower respiratory tract bacterial infections is cefditoren pivoxil (4, 18, 51; Spectracef tablets, package insert [Purdue Pharmaceutical Products]). Cefditoren pivoxil is a broad-spectrum, oral cephalosporin antibacterial with enhanced stability against many common β-lactamases. In vitro studies have demonstrated that cefditoren is highly active against clinically relevant gram-positive and -negative lower respiratory tract organisms, including β-lactamase-producing strains of Haemophilus influenzae and Moraxella catarrhalis (12, 22, 24, 29, 40, 47-49). Cefditoren has also been shown to have highly favorable in vitro activity against penicillin-susceptible and penicillin-intermediate S. pneumoniae (12, 22, 24, 25, 28, 29, 40, 47, 49). It is currently approved in the United States and Europe for the treatment of adults and adolescents with acute exacerbations of chronic bronchitis (AECB) and community-acquired pneumonia (CAP), two of the lower respiratory tract infections most commonly encountered in clinical practice (9, 21, 50).
While the pharmacokinetics of cefditoren have been described (30, 33-35, 38), its pharmacokinetic-pharmacodynamic profile has not been detailed. More importantly, its ability to penetrate into the lung and provide sufficient drug exposure at the site of infection, measured by epithelial lining fluid (ELF) concentrations, has not been well explored. The primary objective of this investigation, therefore, was to characterize the pharmacodynamic profile of cefditoren in plasma and ELF with population pharmacokinetic modeling and Monte Carlo simulation against the array of MICs deemed susceptible, intermediate, and resistant by Clinical and Laboratory Standards Institute (CLSI) for respiratory tract pathogens.
MATERIALS AND METHODS
Patient population.
A total of 24 patients scheduled to undergo fiber-optic bronchoscopy for diagnostic purposes were recruited for this study. The study was started clinically in two centers of internal medicine with pulmonary units. Each patient was randomly assigned to one of the three sampling time windows (1 to 2 h, 2 to 3 h, or 3 to 4 h postadministration). The sampling time was defined by a randomization code provided to the investigator prior to the study initiation. There were 18 male and 6 female patients enrolled, all of them Caucasians. The ages ranged from 35 to 78 years, with an arithmetic mean ± standard deviation of 59 ± 12 years. The weights ranged from 59.8 to 94.9 kg (78.5 ± 8.9 kg). The heights ranged from 166 to 183 cm (173.1 ± 5.0 cm).
The study was approved by the local ethics committee, was conducted according to Good Clinical Practice guidelines (as defined by the International Conference on Harmonisation), and followed all local and national regulatory requirements. Informed consent was obtained from each patient in writing prior to inclusion in the study by a well-qualified investigator.
Pharmacokinetic study design and sampling schedule.
This study was an open, noncontrolled, dual-center, phase I study to assess the concentrations of cefditoren in plasma and ELF after a single oral 400-mg cefditoren dose. Patients received study drug 1 to 2, 2 to 3, or 3 to 4 h prior to bronchoscopy. The subjects received a total dose of 400 mg cefditoren as cefditoren pivoxil together with 240 ml of low-carbonated calcium-poor mineral water at room temperature. To avoid complications of the bronchoscopic procedure, the study drug was administered in the fasting state, after at least 10 h fasting. Blood and bronchoalveolar lavage (BAL) samples (see below) were collected at the same time during the bronchoscopic procedure. Plasma and BAL samples for drug assay were collected in appropriately sized polypropylene tubes, immediately frozen on dry ice, and stored at approximately −70°C.
BAL sampling.
ELF was obtained by BAL (3, 20). During this procedure, 240 to 400 ml of warm isotonic saline was infused in four aliquots into the right middle lobe. The aspirate from the first 50-ml aliquot contains mainly airway fluid and was therefore discarded (46). The remaining three aliquots containing ELF were pooled, the volume was recorded, and the pooled sample was centrifuged immediately (400 × g, 5 min). Immediately after centrifugation, the alveolar macrophages were separated from the supernatant to minimize the efflux of drug from the alveolar macrophages. Determination of study drug concentration in ELF was performed by employing urea as an endogenous marker (43). As urea is an endogenous marker for total body water, we assumed that the concentration of urea in plasma and in ELF is identical. One milliliter of the supernatant was used for determination of urea concentration. The remainder of the supernatant was freeze-dried, reconstituted in an appropriate volume of distilled water, and used for the determination of cefditoren concentration in BAL. For the calculation of cefditoren concentration (C) in the ELF, the following formula was used: CBAL(cefditoren)·[Cserum(urea)/CBAL(urea)].
Determination of cefditoren concentration in plasma by LC-MS/MS.
All pipetting steps were carried out at approximately 4°C. Human plasma (100 μl) sample was deproteinized by addition of 0.200 ml of acetonitrile containing the internal standard (cefotaxime). After thorough mixing, the samples were centrifuged for approximately 10 min at 3,600 rpm at approximately 4°C. Twenty microliters of each sample was chromatographed on a reversed-phase column, eluted with an isocratic solvent system, and monitored by liquid chromatography-tandem mass spectrometry (LC-MS/MS) with a selected reaction monitoring (SRM) method, as follows: precursor → product ion for cefditoren, m/z 507 → m/z 241, and internal standard, m/z 456 → m/z 396; both analyses were in positive mode. Under these conditions, cefditoren and the internal standard were eluted after approximately 1.8 min.
Calibration standards were prepared by adding the appropriate amount of standard solution of cefditoren or the higher-concentration calibration standard to drug-free human plasma. For control of interassay variation, spiked quality controls of cefditoren in human plasma were prepared by adding defined amounts of the stock solution or the spiked control of higher concentration to defined amounts of tested drug-free plasma. No interferences were observed in plasma for cefditoren and the internal standard. Calibration was performed by weighted (1/concentration) linear regression. The linearity of the cefditoren calibration curves in plasma was proven between 0.0100 and 10.0 mg/liter. The quantification limits were identical with the lowest calibration levels. The interday precision and the analytical recovery of the spiked quality control standards of cefditoren in human plasma ranged from 3.4 to 8.9% and were 3.4% (8.00 mg/liter), 4.0% (2.00 mg/liter), 6.6% (0.200 mg/liter), and 8.9% (0.0200 mg/liter). The analytical error in the plasma assay ranged from −7.5 to 3.8% and was 0.3% (8.00 mg/liter), 3.8% (2.00 mg/liter), −7.5% (0.200 mg/liter), and 3.6% (0.0200 mg/liter). The intraday precision of the plasma assay ranged between 1.5 and 3.9% and was 2.3% (8.00 mg/liter), 2.1% (2.00 mg/liter), 1.5% (0.200 mg/liter) and 3.9% (0.0200 mg/liter). The analytical error of the plasma assay ranged from −0.8 to 3.7% and was 0.3% (8.00 mg/liter), −0.8% (2.00 mg/liter), 1.2% (0.200 mg/liter) and 3.7% (0.0200 mg/liter).
Determination of cefditoren concentration in BAL by LC-MS/MS.
Human BAL (1 ml) was mixed with 100 μl methanol. A 20-μl portion of each sample was chromatographed on a reversed-phase column, eluted with an isocratic solvent system, and monitored by LC-MS/MS with an SRM method as follows: precursor → product ion for cefditoren, m/z 507 → m/z 241, and internal standard, m/z 456 → m/z 396; both analyses were in positive mode. Under these conditions, cefditoren and the internal standard (cefotaxime) were eluted after approximately 1.2 min.
Calibration standards were prepared by adding the appropriate amount of standard solution of cefditoren or the higher-concentration calibration standard to a buffer solution. For control of interassay variation, spiked quality controls in a buffer solution of cefditoren were prepared by adding defined amounts of the stock solution or the spiked control of higher concentration to defined amounts of buffer solution. No interferences were observed for cefditoren and the internal standard. Calibration was performed by weighted (1/concentration) linear regression. The linearity of the cefditoren calibration curves in buffer solution was proven between 0.00100 and 0.400 mg/liter. The quantification limits were identical with the lowest calibration levels. The interday precision and the analytical recovery of the spiked quality control standards of cefditoren in human plasma ranged from 4.0 to 6.6% and were 4.0% (0.800 mg/liter), 4.6% (0.200 mg/liter), 6.2% (0.0200 mg/liter), and 6.6% (0.00200 mg/liter). The analytical error in the bronchoalveolar fluid assay ranged from −4.3 to 6.6% and was 6.6% (0.800 mg/liter), 4.9% (0.200 mg/liter), −4.3% (0.0200 mg/liter), and 3.3% (0.00200 mg/liter). The intraday precision of the bronchoalveolar fluid assay ranged between 2.3 and 7.9% and was 2.3% (0.800 mg/liter), 6.9% (0.200 mg/liter), 7.9% (0.0200 mg/liter) and 5.2% (0.00200 mg/liter). The analytical error of the bronchoalveolar fluid assay ranged from −5.0 to 4.7% and was 4.7% (0.800 mg/liter), 1.0% (0.200 mg/liter), −3.4% (0.0200 mg/liter) and −5.0% (0.00200 mg/liter).
Determination of urea in BAL by LC-MS/MS.
To 1 ml of each human BAL sample, 100 μl of the internal standard solution in Milli-Q-water and 1 ml methanol were added. A 10-μl portion of each sample was chromatographed on a reversed-phase column, eluted with an isocratic solvent system, and monitored by LC-MS/MS with an SRM method as follows: precursor → product ion for urea, m/z 157 → m/z 114, and internal standard, m/z 160 → m/z 115; both analyses were in positive mode. Under these conditions, urea and the internal standard were eluted after approximately 2.3 min.
Calibration standards were prepared by adding the appropriate amount of standard solution of urea or the higher-concentration calibration standard. For control of interassay variation, spiked quality controls of urea were prepared by adding defined amounts of the stock solution or the spiked control of higher concentration. No interferences were observed for urea and the internal standard. Calibration was performed by weighted (1/concentration) linear regression. The linearity of the urea calibration curves was proven between 0.208 and 8.00 mg/liter. The quantification limits were identical with the lowest calibration levels. The interday precision and the analytical recovery of the spiked quality control standards of urea in human plasma ranged from 0.3 to 0.9% and were 0.3% (8.00 mg/liter), 0.9% (1.60 mg/liter) and 0.3% (0.400 mg/liter). The analytical error was −1.4% (8.00 mg/liter), 4.2% (1.60 mg/liter), and 8.6% (0.400 mg/liter).
Population pharmacokinetic modeling methods.
All data were analyzed in a population pharmacokinetic model using the big nonparametric adaptive grid with adaptive γ (BigNPAG) program of Leary, Jelliffe, Schumitzky, and van Guilder (29a). The pharmacokinetic model was parameterized as a four-compartment model with first-order absorption from the absorption compartment and with elimination and transfer from the central compartment modeled as first-order processes.
The general differential equations for the model are as follows: dX(1)/dt = −ka·X(1); dX(2)/dt = ka·X(1) − [(CL/VC) + k23+ k24)·X(2) + k32·X(3) + k42·X(4)]; dX(3)/dt = k23·X(2) − k32·X(3); dX(4)/dt = k24·X(2) − k42·X(4). ka is the absorption rate constant (per hour); X(1) is the amount of drug in the absorption compartment (in milligrams); X(2) is the amount of drug in the central compartment (in milligrams); X(3) is the amount of drug in the peripheral compartment (in milligrams); X(4) is the amount of drug in the ELF compartment (in milligrams); CL/F is the apparent clearance from the central compartment (liters per hour); k23, k32, k24, and k42 are first-order intercompartmental transfer rate constants (per hour); and VC/F is a scalar term and represents the apparent volume of the central compartment (in liters). F is the extent of absorption after oral dosing. Not shown is VELF/F, which is a scalar term and is the apparent volume of the ELF.
The inverse of the estimated assay variance was used as the first estimate for weighting in the pharmacokinetic modeling. Weighting was accomplished by making the assumption that total observation variance was proportional to assay variance. Assay variance was determined on a between-day basis.
Upon attaining convergence, Bayesian estimates for each patient were obtained using the “population of one” utility within BigNPAG. The mean, median, and modal values were employed as measures of central tendency for the population parameter estimates and were evaluated in the Bayesian analysis. Scatter plots were examined for individual patients and for the population as a whole. Goodness of fit was assessed by regression with an observed-predicted plot, coefficients of determination, and log likelihood values. Predictive performance evaluation was based on weighted mean error and the bias-adjusted weighted mean squared error.
Monte Carlo simulation.
The median parameter vector and major diagonal from the population pharmacokinetic model were embedded in subroutine PRIOR of the ADAPT II package of programs of D'Argenio and Schumitzky (17). The median parameter vector was selected as the measure of central tendency because it provided the best goodness of fit. The full covariance matrix could not be employed because it did not have symmetric positive definite properties. The population simulation without process noise option was employed. A 9,999-subject Monte Carlo simulation (maximum number allowed by program) was performed for cefditoren (400 mg orally every 12 h). Both normal and log normal distributions were evaluated, and these were discriminated on their ability to recreate the original mean parameter values and corresponding standard deviations from the population analyses. The median parameter values from the population pharmacokinetic model were employed to simulate steady-state concentrations (48 h after the start of dosing) and to generate plasma and ELF concentration-time curves. The plasma pharmacokinetic data for cefditoren were adjusted for 88% protein binding to reflect unbound drug concentrations in the data analysis (Spectracef package insert; Purdue Pharmaceutical Products). The ELF pharmacokinetic data were not adjusted for protein binding because the protein binding of cefditoren in the ELF is currently unknown. The fraction of simulated subjects who achieved times for which plasma concentration exceeded the MIC of the organism for both plasma (free drug) (fT>MIC) and ELF (total drug) (T>MIC) of 33% and 50 to 70% of the dosing interval was calculated for the range of MICs from 0.006 mg/liter to 1 mg/liter. This range of MICs was examined because the objective of this analysis was to examine the probability of target attainment in relation to the current CLSI breakpoints for S. pneumoniae and H. influenzae: susceptible, ≤0.125 mg/liter; intermediate, 0.25 mg/liter; and resistant, ≥0.5 mg/liter. The T>MIC pharmacodynamics endpoints of 33% and 60 to 70% were selected because they have been identified as the targets for bacteriostasis and bactericidal killing (13-16). Additionally, a T>MIC of 50% was examined because this endpoint is used by the CLSI to assess cephalosporin susceptibility breakpoints.
Monte Carlo simulation was used to calculate the mean and median ELF-to-plasma penetration ratios by estimating the area under the concentration-time curves for ELF and plasma from zero to infinity (AUCELF0-∞ and AUCplasma0-∞) after a single simulated dose and computing the ratio. The penetration ratio derived from the mean parameter vector from the population model was also calculated. Systat for Windows (version 10.2) was used for all data transformation.
RESULTS
Pharmacokinetic results.
Summary statistics for the plasma and ELF concentrations are displayed in Table 1. Arithmetic mean concentrations of cefditoren in ELF were 38.1 ± 50.1% of the plasma concentrations within the 1- to 2-h sampling interval, 23.2 ± 18.1% within the 2- to 3-h interval, and 31.8 ± 19.2% within the 3- to 4-h interval. It is important to note that the mean and standard deviation for each interval were calculated separately for plasma and ELF. At each interval, the ratio was also calculated. Thus, the mean of the ratios would not be expected to be the same as the ratio of the means.
TABLE 1.
Summary statistics of cefditoren plasma and ELF concentrations after a single oral administration of 400 mg cefditoren as cefditoren pivoxil
| Collection interval (h) | Concn (mg/liter) in:
|
Ratio (%) | |
|---|---|---|---|
| Plasma | ELF | ||
| 1.0-2.0 | 1.78 ± 1.27 | 0.39 ± 0.21 | 38.1 ± 50.1 |
| 2.01-3.0 | 1.33 ± 0.95 | 0.34 ± 0.25 | 23.2 ± 18.1 |
| 3.01-4.0 | 1.03 ± 0.51 | 0.30 ± 0.18 | 31.8 ± 19.2 |
Population pharmacokinetic modeling.
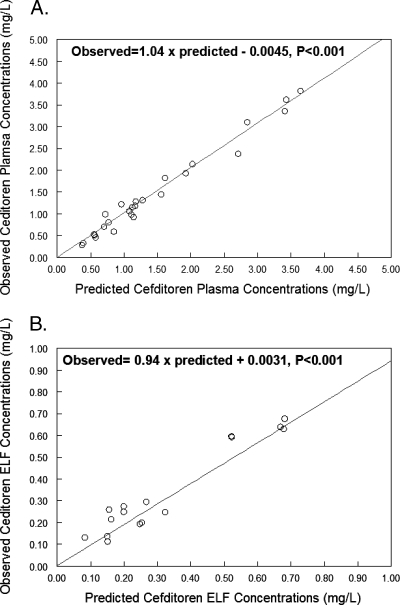
The population parameter estimates identified by BigNPAG for the pharmacokinetic model are displayed in Table 2. Using the population median parameter values as the measure of central tendency, the overall fit of the model to the data was good and the observed-predicted plots for plasma and ELF after the Bayesian step were highly acceptable. For plasma, r2 was 0.98 and the observed-predicted plot showed the following best-fit regression line: observed = 1.04 × predicted − 0.0045 (Fig. 1A). The mean weighted error (measure of bias) for plasma was −0.01 mg/liter, and the bias-adjusted weighted mean squared error (measure of precision) was 0.45 (mg/liter)2. For ELF, r2 was 0.93, and the observed-predicted plot showed the following best-fit regression line: observed = 0.94 × predicted + 0.0031 (Fig. 1B). The mean weighted error (measure of bias) for ELF was −0.08 mg/liter, and the bias-adjusted weighted mean squared error (measure of precision) was 0.19 (mg/liter)2.
TABLE 2.
Population phamacokinetic parameters obtained by BigNPAG
| Calculation | VC/F (liters) | CL/F (liters/h) | k23 (h−1) | k32 (h−1) | k24 (h−1) | k42 (h−1) | VELF/F (liters) | Ka (h−1) |
|---|---|---|---|---|---|---|---|---|
| Mean | 36.13 | 61.70 | 16.69 | 14.26 | 10.43 | 18.03 | 65.24 | 3.80 |
| Median | 43.32 | 42.99 | 16.48 | 9.24 | 7.20 | 17.83 | 72.71 | 1.35 |
| SD | 13.86 | 25.39 | 8.88 | 8.87 | 7.66 | 7.97 | 28.59 | 2.86 |
FIG. 1.
Observed versus predicted plots in plasma (A) and ELF (B).
Monte Carlo simulation.
A 9,999-subject Monte Carlo simulation was performed for both the plasma and ELF data to estimate the target attainment probabilities in both the plasma and ELF. Log normal distributions were selected for the population simulation based on their ability to recapitulate the original mean parameter values and corresponding standard deviations.
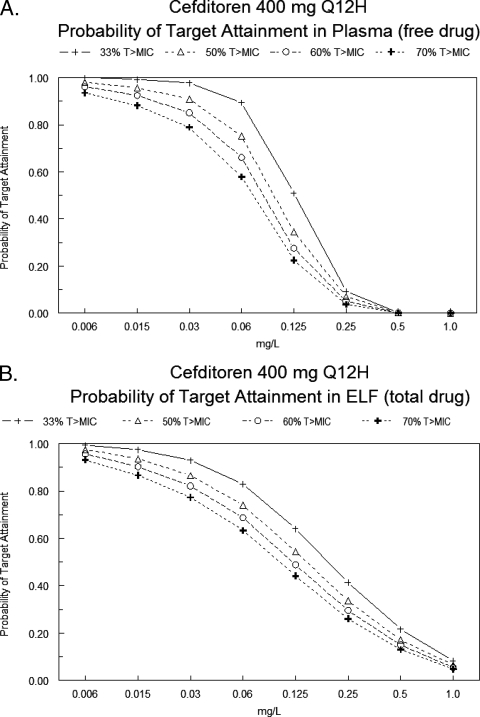
The results of probability of target attainment analysis in plasma and ELF for cefditoren (400 mg orally every 12 h) is displayed in Fig. 2. In plasma, the probability of achieving an fT>MIC of 33% was >90% for MICs of ≤0.03 mg/liter. For a MIC of 0.06 mg/liter, there was an 89.48% probability of achieving an fT>MIC of 33%. For an fT>MIC of 50%, the probability of target attainment was 90.82% at a MIC of 0.03 mg/liter, and the probability of target attainment was significantly less for higher MICs. The probability of achieving fT>MIC of 60 to 70% was <90% for MICs of ≥0.03 mg/liter (Fig. 2A).
FIG. 2.
Target attainment analysis of cefditoren (400 mg orally every 12 h) for plasma (free drug) (A) and ELF (total drug) (B).
The probability of target attainment in ELF was similar to plasma (Fig. 2B). The probability of achieving a T>MIC of 33% was >90% for MICs of ≤0.03 mg/liter. For a MIC of 0.06 mg/liter, the probability of achieving a T>MIC of 33% was 82.95%. For a T>MIC of 50%, the probability of target attainment was 86.51% at a MIC of 0.03 mg/liter and was significantly less for higher MICs. The probabilities of achieving T>MIC of 60% and 70% were 90.26% and 86.65%, respectively, at a MIC of 0.0125 mg/liter and were significantly less for higher MICs.
The mean (standard deviation) AUCELF0-∞ and AUCplasma0-∞ were 4.15 (7.72) mg·h/liter and 12.64 (7.55) mg·h/liter, respectively. The mean ± standard deviation AUCELF/AUCplasma penetration ratio was 0.33 ± 0.48. The median (25th and 75th percentile) AUCELF0-∞ and AUCplasma0-∞ were 1.97 (0.88 to 4.40) mg·h/liter and 10.83 (7.48 to 15.74) mg·h/liter, respectively. The median AUCELF/AUCplasma penetration ratio was 0.18, and the 25th and 75th percentiles were 0.09 and 0.38, respectively. The average value for the Monte Carlo simulation is skewed because of outliers, as is evident when one examines the median value of the penetration ratio of 0.18, the mean ratio of 0.33, and the large standard deviation of 0.48. The AUCELF/AUCplasma penetration ratio derived from the mean parameter vector from the population model was 0.24 and further reflects the influence of outliers on the mean penetration ratio from the Monte Carlo simulation.
DISCUSSION
Treatment of lower respiratory tract infections has been compromised by the development of resistance, particularly multidrug resistance in S. pneumoniae. Over the past 20 years, rates of penicillin resistance in S. pneumoniae have dramatically increased. Many of these penicillin-resistant strains are often cross-resistant to many other classes of antibiotics (1, 5, 8, 10, 22, 23, 44, 45, 48, 52). Cefditoren is a new broad-spectrum oral cephalosporin with good activity against respiratory tract pathogens, including penicillin-susceptible and -intermediate strains of S. pneumoniae (12, 22, 24-29, 40, 47-49). Its bacterial activity, measured by MIC, is similar or superior to that of many other commonly used cephalosporins (12, 22, 24, 25, 28, 29, 40, 48).
While the MIC is a useful quantitative determination of in vitro antibacterial activity, it suffers from many notable limitations. For example, the MIC does not account for the following: time course of antimicrobial activity (drug concentrations change during dosing interval), rate of bacterial killing, dose-kill response relationship, and postantibiotic effect. For antimicrobials, integration of pharmacokinetic parameters and MIC surmounts many of the limitations of the MIC and has been shown to be a more reliable predictor of clinical and microbiologic outcomes (13-15, 19, 42). For β-lactams, in vitro and animal studies have demonstrated that the fT>MIC appears to be the best predictor of bacterial killing and outcomes (13-15). The free beta-lactam concentrations in plasma do not have to remain above the MIC for the entire dosing interval, and the fraction of the dosing interval required for maximal bacterial effect varies for the different types of beta-lactams. Although the precise fT>MIC varies for different drug-bacterium combinations, cephalosporins achieve a static effect when the free (unbound) concentration remains above the MIC of the organisms for 30 to 40% of the dosing interval, and a near-maximal bactericidal effect is achieved for an fT>MIC of 60 to 70%. These endpoints are slightly lower for the penicillins and carbapenems (13-15, 19).
Population pharmacokinetic modeling and Monte Carlo simulation were used to describe the pharmacodynamic profile of cefditoren in the both plasma and ELF. Since antibiotic delivery to the site of infection is imperative for optimal therapy, it is extremely important to be able to accurately estimate the ability of drugs to penetrate the infected site and achieve sufficient concentrations for the desired endpoint. For extracellular respiratory tract pathogens like S. pneumoniae and H. influenzae, determination of drug concentration in ELF is currently the best estimate for ascertaining the degree of drug exposure for these organisms.
Most often, analysis of ELF penetration data is limited to obtaining ratios of drug concentrations in the ELF to those determined simultaneously in plasma. As the drug has to penetrate from plasma to ELF, these ratios will change as a function of time, as observed in this study (Table 1). This phenomenon, known as system hysteresis, makes examination of single-time-point penetration ratios suboptimal, because the estimates of drug penetration will strongly depend on the sampling time, as is the case here, where the penetration ratios changed from 23.2 to 38.1%. Population pharmacokinetic modeling is able to surmount this limitation because of its ability to estimate population pharmacokinetics and their associated dispersions for subjects with minimal sampling times. Once the population pharmacokinetics are estimated, Monte Carlo simulation can be used to estimate the ability of a drug to penetrate the site of infection and to characterize its ability to achieve the desired pharmacodynamic endpoint at that site.
The results indicate that cefditoren penetrates reasonably well into the ELF, as defined by the mean AUCELF/AUCplasma penetration ratio. Specifically, the AUCELF/AUCplasma penetration ratio (mean ± standard deviation) was 0.33 ± 0.48, and the median AUCELF/AUCplasma penetration ratio was 0.18 (25th and 75th percentile values were 0.09 and 0.38, respectively). When one considers that the plasma protein binding of cefditoren is approximately 88%, the total-drug AUC in ELF was higher than the free-drug AUC in plasma. Part of the explanation for this finding is that we employed total-drug AUC for ELF. It is currently unknown what the protein binding would be in ELF, but this could help explain this finding. The overall probability of target attainment in plasma and ELF, however, was suboptimal. The probability of achieving a bacteriostatic effect (fT>MIC, 33%) was <90% in both plasma and ELF for a range of MICs considered susceptible by CLSI; the CLSI breakpoint for susceptibility is ≤0.125 mg/liter. More importantly, the probability of achieving a T>MIC of 50%, which is the current pharmacodynamic target used by the CLSI in the assessment of cephalosporin susceptibility breakpoints, was <90% in both plasma and ELF for MICs of >0.03 mg/liter.
The low probability of target attainment is most likely related to its apparent clearance, protein binding, and extent of absorption. In this study, the median apparent clearance was 42.99 liters/h. While this estimate initially appears to be high, the mean plasma AUC0-∞ after a 400-mg cefditoren dose, calculated from the simulated plasma concentration, was 12.64 ± 7.55 mg·h/liter, and this is highly consistent with prior estimates of average AUC0-12 for cefditoren (30, 33-35, 37, 38). Previous estimates of plasma AUC0-∞ have ranged from 10 to 15 mg·h/liter.
Another factor for the low observed probability of target attainment in plasma is its high protein binding. The mean binding of cefditoren to plasma protein is 88% (Spectracef package insert; Purdue Pharmaceutical Products). While the relationship between protein binding and microbiological activity has been highly debated, there are considerable data demonstrating that protein binding adversely impacts microbiological outcomes and that only free or unbound drug is microbiologically active (7, 36; Spectracef package insert; Purdue Pharmaceutical Products). Based on these studies, only free drug in plasma was studied. Total drug was studied in ELF, because the influence of protein binding in ELF has not been studied. Further study is needed to delineate the influence of protein binding in ELF. The presence of protein binding in ELF would result in lower observed probability of target attainment in ELF.
Another explanation for the low probability of target attainment is that the cefditoren pharmacokinetics samples were obtained under fasting conditions. The present study had to be performed in the fasting state, since bronchoscopy must not be performed in the fed state, particularly after a high-fat meal. The absolute bioavailability of cefditoren under fasting conditions is approximately 14% (Spectracef package insert; Purdue Pharmaceutical Products). The administration of cefditoren after a high-fat meal is associated with a very strong increase in maximal plasma concentration and maximal AUCplasma 50% and 70% increases, respectively (30; Spectracef package insert; Purdue Pharmaceutical Products). Therefore, administration with food would improve the observed probability of target attainment estimates by approximately 1 dilution and thus provide higher probabilities of target attainment for the range of MICs deemed susceptible by the CLSI (≤0.125 mg/liter).
Given the low probability of target attainment for MICs of ≥0.125 mg/liter, one would expect low clinical success rates. This, however, has not been the case, and cefditoren has performed extremely well in AECB and CAP clinical trials (9, 21, 50). In these trials, the majority of the organisms recovered had MICs of ≤0.125 mg/liter, and the probability of target attainment analysis suggests that cefditoren has a high probability of achieving a T>MIC of 33% in both plasma (free-drug concentration) and ELF (total-drug concentration). In addition, administration with a high-fat meal would provide high probabilities of target attainment for the range of MICs deemed susceptible by the CLSI (≤0.125 mg/liter). Currently, there are insufficient clinical data to determine if cefditoren is effective against higher-MIC pathogens. It is also important to note that the pharmacodynamic targets for bacteriostasis (T>MIC, 30 to 40%) and bactericidal activity (T>MIC, 60 to 70%) were originally developed in neutropenic-animal models (14), and restoration of granulocytes has been shown to decrease the magnitude of exposure necessary for bacteriostatic and/or bactericidal effect (2). One could postulate that in immunocompetent humans, like the patients studied in the cefditoren clinical trials, the patients' intact immune systems can assist in clearing the infection, and the drug exposure requirements would be less than targets derived from neutropenic-animal models.
In summary, cefditoren penetrated well into the ELF but had a suboptimal probability of target attainment profile in both plasma and ELF. The probability of achieving a bacteriostatic response was <90% for MICs of >0.03 mg/liter in both plasma and ELF. An increased extent of absorption under the influence of food would increase this MIC cutoff value, whereas protein binding in ELF would decrease it. This finding is cause for concern, given the currently used CLSI breakpoint of 0.125 mg/liter. Despite these findings, cefditoren has performed reasonably well in AECB and CAP clinical trials. It is important to note that these studies involved immunocompetent adults, and pathogens recovered from these studies had low MICs. Currently, there is insufficient clinical data to determine if cefditoren is effective against higher-MIC pathogens. Further study is needed to determine its activity against higher-MIC pathogens in humans.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Almirall, J., I. Bolibar, J. Vidal, G. Sauca, P. Coll, B. Niklasson, M. Bartolome, and X. Balanzo. 2000. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur. Respir. J. 15:757-763. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., and W. A. Craig. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 46:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The BAL Cooperative Group Steering Committee. 1990. Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am. Rev. Respir. Dis. 141:S169-S202. [DOI] [PubMed] [Google Scholar]
- 4.Balbisi, E. A. 2002. Cefditoren, a new aminothiazolyl cephalosporin. Pharmacotherapy 22:1278-1293. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, Jr., D. M. Musher, and M. J. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett, J. G., and L. M. Mundy. 1995. Community-acquired pneumonia. N. Engl. J. Med. 333:1618-1624. [DOI] [PubMed] [Google Scholar]
- 7.Bilello, J. A., P. A. Bilello, K. Stellrecht, J. Leonard, D. W. Norbeck, D. J. Kempf, T. Robins, and G. L. Drusano. 1996. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 40:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, S. D., and M. J. Rybak. 2004. Antimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001-2002, as part of the PROTEKT US study. J. Antimicrob. Chemother. 54(Suppl. 1):i7-i15. [DOI] [PubMed] [Google Scholar]
- 9.Bucko, A. D., B. J. Hunt, S. L. Kidd, and R. Hom. 2002. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg BID with either cefuroxime 250 mg BID or cefadroxil 500 mg BID for the treatment of uncomplicated skin and skin-structure infections. Clin. Ther. 24:1134-1147. [DOI] [PubMed] [Google Scholar]
- 10.Castanheira, M., A. C. Gales, R. E. Mendes, R. N. Jones, and H. S. Sader. 2004. Antimicrobial susceptibility of Streptococcus pneumoniae in Latin America: results from five years of the SENTRY Antimicrobial Surveillance Program. Clin. Microbiol. Infect. 10:645-651. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 1997. Introduction to Table V. Premature deaths, monthly mortality, and monthly physician contacts—United States. Morb. Mortal. Wkly. Rep. 46:556-561. [PubMed] [Google Scholar]
- 12.Clark, C. L., K. Nagai, B. E. Dewasse, G. A. Pankuch, L. M. Ednie, M. R. Jacobs, and P. C. Appelbaum. 2002. Activity of cefditoren against respiratory pathogens. J. Antimicrob. Chemother. 50:33-41. [DOI] [PubMed] [Google Scholar]
- 13.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 14.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 15.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 16.Craig, W. A., and D. R. Andes. 2000. In vivo pharmacodynamic activity of cefditoren against Streptococcus pneumoniae, poster 2248. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother.
- 17.D'Argenio, D. Z., and A. Schumitzky. 1979. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput. Programs Biomed. 9:115-134. [DOI] [PubMed] [Google Scholar]
- 18.Darkes, M. J., and G. L. Plosker. 2002. Cefditoren pivoxil. Drugs 62:319-336, 337-338. [DOI] [PubMed] [Google Scholar]
- 19.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 20.European Society of Pneumology Task Group. 1989. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur. Respir. J. 2:561-585. [PubMed] [Google Scholar]
- 21.Fogarty, C. M., M. Cyganowski, W. A. Palo, R. C. Hom, and W. A. Craig. 2002. A comparison of cefditoren pivoxil and amoxicillin/ clavulanate in the treatment of community-acquired pneumonia: a multicenter, prospective, randomized, investigator-blinded, parallel-group study. Clin. Ther. 24:1854-1870. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2000. Susceptibility of Streptococcus pneumoniae and Haemophilus influenzae to cefditoren, and provisional interpretive criteria. Diagn. Microbiol. Infect. Dis. 37:265-269. [DOI] [PubMed] [Google Scholar]
- 23.Hoban, D. J., D. J. Biedenbach, A. H. Mutnick, and R. N. Jones. 2003. Pathogen of occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY Antimicrobial Surveillance Study (2000). Diagn. Microbiol. Infect. Dis. 45:279-285. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, D. M., D. J. Biedenbach, M. L. Beach, M. A. Pfaller, and R. N. Jones. 2000. Antimicrobial activity and in vitro susceptibility test development for cefditoren against Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus species. Diagn. Microbiol. Infect. Dis. 37:99-105. [DOI] [PubMed] [Google Scholar]
- 25.Jones, R. N., D. J. Biedenbach, M. A. Croco, and M. S. Barrett. 1998. In vitro evaluation of a novel orally administered cephalosporin (Cefditoren) tested against 1249 recent clinical isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 31:573-578. [DOI] [PubMed] [Google Scholar]
- 26.Jones, R. N., D. J. Biedenbach, and D. M. Johnson. 2000. Cefditoren activity against nearly 1000 non-fastidious bacterial isolates and the development of in vitro susceptibility test methods. Diagn. Microbiol. Infect. Dis. 37:143-146. [DOI] [PubMed] [Google Scholar]
- 27.Karlowsky, J. A., I. A. Critchley, D. C. Draghi, M. E. Jones, C. Thornsberry, and D. F. Sahm. 2002. Activity of cefditoren against beta-lactamase-positive and -negative Haemophilus influenzae and Moraxella catarrhalis. Diagn. Microbiol. Infect. Dis. 42:53-58. [DOI] [PubMed] [Google Scholar]
- 28.Karlowsky, J. A., M. E. Jones, D. C. Draghi, I. A. Critchley, C. Thornsberry, and D. F. Sahm. 2002. In vitro susceptibility of recent clinical isolates of pneumococci to the investigational cephalosporin cefditoren. Diagn. Microbiol. Infect. Dis. 42:59-64. [DOI] [PubMed] [Google Scholar]
- 29.Kelly, L. M., M. R. Jacobs, and P. C. Appelbaum. 1999. Comparison of agar dilution, microdilution, E-test, and disk diffusion methods for testing activity of cefditoren against Streptococcus pneumoniae. J. Clin. Microbiol. 37:3296-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Leary, R., R. Jelliffer, A. Schumitzky, and M. van Guilder. 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p. 389-394. Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems. IEEE Computer Society, Bethesda, MD.
- 30.Li, J. T., F. Hou, H. Lu, T. Y. Li, and H. Li. 1997. Phase I clinical trial of cefditoren pivoxil (ME 1207): pharmacokinetics in healthy volunteers. Drugs Exp. Clin. Res. 23:145-150. [PubMed] [Google Scholar]
- 31.Mandell, L. A., J. G. Bartlett, S. F. Dowell, T. M. File, Jr., D. M. Musher, and C. Whitney. 2003. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37:1405-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marston, B. J., J. F. Plouffe, T. M. File, Jr., B. A. Hackman, S. J. Salstrom, H. B. Lipman, M. S. Kolczak, and R. F. Breiman. 1997. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch. Intern. Med. 157:1709-1718. [PubMed] [Google Scholar]
- 33.Mayer, M., M. D., and G. Witt. 2000. Effect of an H2 receptor antagonist or an antacid on the pharmacokinetics of cefditoren, poster 313. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother.
- 34.Mayer, M., M. D., and G. Witt. 2000. Effect of hepatic impairment on the pharmacokinetics of cefditoren, poster 312. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother..
- 35.Mayer, M., M. D., and G. Witt. 2000. Pharmacokinetics of cefditoren in blister fluid and plasma, abstr. 656. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother.
- 36.Merrikin, D. J., J. Briant, and G. N. Rolinson. 1983. Effect of protein binding on antibiotic activity in vivo. J. Antimicrob. Chemother. 11:233-238. [DOI] [PubMed] [Google Scholar]
- 37.Mulford, D., M. M., and G. Witt. 2000. Effect of age and gender on the pharmacokinetics of cefditoren, poster 310. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother.
- 38.Mulford, D., M. M., and G. Witt. 2000. Effect of renal impairment on the pharmacokinetics of cefditoren, poster 311. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother.
- 39.Niederman, M. S., J. S. McCombs, A. N. Unger, A. Kumar, and R. Popovian. 1998. The cost of treating community-acquired pneumonia. Clin. Ther. 20:820-837. [DOI] [PubMed] [Google Scholar]
- 40.Peric, M., F. A. Browne, M. R. Jacobs, and P. C. Appelbaum. 2003. Activity of nine oral agents against gram-positive and gram-negative bacteria encountered in community-acquired infections: use of pharmacokinetic/pharmacodynamic breakpoints in the comparative assessment of beta-lactam and macrolide antimicrobial agents. Clin. Ther. 25:169-177. [DOI] [PubMed] [Google Scholar]
- 41.Pinner, R. W., S. M. Teutsch, L. Simonsen, L. A. Klug, J. M. Graber, M. J. Clarke, and R. L. Berkelman. 1996. Trends in infectious diseases mortality in the United States. JAMA 275:189-193. [PubMed] [Google Scholar]
- 42.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 43.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 44.Samore, M. H., M. K. Magill, S. C. Alder, E. Severina, L. Morrison-De Boer, J. L. Lyon, K. Carroll, J. Leary, M. B. Stone, D. Bradford, J. Reading, A. Tomasz, and M. A. Sande. 2001. High rates of multiple antibiotic resistance in Streptococcus pneumoniae from healthy children living in isolated rural communities: association with cephalosporin use and intrafamilial transmission. Pediatrics 108:856-865. [DOI] [PubMed] [Google Scholar]
- 45.Schrag, S. J., L. McGee, C. G. Whitney, B. Beall, A. S. Craig, M. E. Choate, J. H. Jorgensen, R. R. Facklam, and K. P. Klugman. 2004. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrob. Agents Chemother. 48:3016-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, S. F., A. Guz, A. J. Winning, N. T. Cooke, G. H. Burton, and T. D. Tetley. 1988. Comparison of human lung surface protein profiles from the central and peripheral airways sampled using two regional lavage techniques. Eur. Respir. J. 1:792-800. [PubMed] [Google Scholar]
- 47.Soriano, F., J. J. Granizo, P. Coronel, M. Gimeno, E. Rodenas, M. Gracia, C. Garcia, R. Fernandez-Roblas, J. Esteban, and I. Gadea. 2004. Antimicrobial susceptibility of Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis isolated from adult patients with respiratory tract infections in four southern European countries. The ARISE project. Int. J. Antimicrob. Agents 23:296-299. [DOI] [PubMed] [Google Scholar]
- 48.Soriano, F., J. J. Granizo, A. Fenoll, M. Gracia, R. Fernandez-Roblas, J. Esteban, I. Gadea, P. Coronel, M. Gimeno, E. Rodenas, and F. Santos. 2003. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae isolated in four southern European countries (ARISE project) from adult patients: results from the cefditoren surveillance program. J. Chemother. 15:107-112. [DOI] [PubMed] [Google Scholar]
- 49.Thornsberry, C., J. A. Karlowsky, L. J. Kelly, D. C. Draghi, I. A. Critchley, M. E. Jones, and D. F. Sahm. 2001. Comparative activity of cefditoren and other oral beta-lactams against nonpneumococcal streptococci. Chemotherapy 47:332-343. [DOI] [PubMed] [Google Scholar]
- 50.van Zyl, L., J. G. le Roux, J. A. LaFata, R. S. Volk, W. A. Palo, R. Flamm, and R. C. Hom. 2002. Cefditoren pivoxil versus cefpodoxime proxetil for community-acquired pneumonia: results of a multicenter, prospective, randomized, double-blind study. Clin. Ther. 24:1840-1853. [DOI] [PubMed] [Google Scholar]
- 51.Wellington, K., and M. P. Curran. 2004. Cefditoren pivoxil: a review of its use in the treatment of bacterial infections. Drugs 64:2597-2618. [DOI] [PubMed] [Google Scholar]
- 52.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]