Abstract
We investigated the in vitro activities of posaconazole (POS), fluconazole (FLC), amphotericin B (AMB), and caspofungin (CAS) against four clinical isolates of Candida glabrata with various susceptibilities to FLC (FLC MICs ranging from 1.0 to >64 μg/ml). POS MICs ranged from ≤0.03 to 0.5 μg/ml; AMB MICs ranged from 0.25 to 2.0 μg/ml, while CAS MICs ranged from 0.03 to 0.25 μg/ml. When FLC MICs increased, so did POS MICs, although we did not observe any isolate with a POS MIC greater than 0.5 μg/ml. Time-kill experiments showed that POS, FLC, and CAS were fungistatic against all isolates, while AMB at eight times the MIC was fungicidal against three out of four isolates of C. glabrata tested. Then, we investigated the activity of POS in an experimental model of disseminated candidiasis using three different isolates of C. glabrata: one susceptible to FLC (S; FLC MICs ranging from 1.0 to 4.0 μg/ml; POS MIC of ≤0.03 μg/ml), one susceptible in a dose-dependent manner (SDD; FLC MICs ranging from 32 to 64 μg/ml; POS MICs ranging from 0.125 to 0.25 μg/ml), and another one resistant to FLC (R; FLC MIC of >64 μg/ml; POS MIC of 0.5 μg/ml). FLC significantly reduced the kidney burden of mice infected with the S strain (P = 0.0070) but not of those infected with the S-DD and R strains. POS was significantly effective against all three isolates at reducing the kidney fungal burden with respect to the controls (P ranging from 0.0003 to 0.029). In conclusion, our data suggest that POS may be a useful option in the management of systemic infections caused by C. glabrata. Additionally, the new triazole may be a therapeutic option in those cases where an FLC-resistant isolate is found to retain a relatively low POS MIC.
In recent years, the patterns of Candida infections have changed. Previously, Candida albicans was the most prevalent cause of Candida infections, whereas in recent times, other Candida species have become common pathogens causing infections (11, 12).
Candida glabrata has recently emerged as the second most common cause of invasive candidiasis, and there are increasing numbers of reports showing its important role in determining either superficial or deep-seated infections (11, 12). Systemic infections due to C. glabrata are characterized by a high mortality rate, and they are difficult to treat due to a reduced susceptibility of this species to azole drugs, especially to fluconazole (FLC) (11, 12).
Posaconazole (POS) has been developed mainly to combat the development of resistance to azoles in yeasts, in particular to FLC, and to expand the spectrum of susceptible pathogens (5-7, 17-22). Numerous in vitro studies demonstrated that POS has a broad spectrum of activity against the majority of yeasts, filamentous fungi, and azole-resistant Candida species (2, 9, 19). The new triazole exerts the same mechanism of action as does the other azole derivatives (i.e., it inhibits ergosterol production by binding and inhibiting the lanosterol 14α-demethylase) (4, 19).
In the present study, we investigated the in vitro and in vivo activities of POS against clinical isolates of C. glabrata with various susceptibilities to FLC.
MATERIALS AND METHODS
Isolates.
Four clinical isolates of C. glabrata were used in this study. The isolates were recovered from blood. Each strain represented a unique isolate from a patient. Yeast isolates were identified at the species level by conventional morphological and biochemical methods and stored at −70°C in 10% glycerol. Before the initiation of the study, yeast isolates were subcultured on Sabouraud dextrose agar plates to ensure viability and purity. Candida parapsilosis ATCC 22019 was used as the quality control.
Drugs.
POS (Schering-Plough Research Institute, Kenilworth, NJ) was prepared with polyethylene glycol 200 (Janssen Chimica, Geel, Belgium) for both in vitro and in vivo studies. A stock solution of FLC (Pfizer Inc.) was prepared in sterile saline solution for both in vitro and in vivo studies. Caspofungin (CAS) was used as a commercial preparation (Cancidas; Merck Sharp & Dohme) for both in vitro and in vivo experiments. It was dissolved following the manufacturer's instructions. Amphotericin B (AMB) was used as pure powder (Sigma) for in vitro studies and as a commercial preparation (Fungizone; Bristol-Myers Squibb) for in vivo studies. It was dissolved in dimethyl sulfoxide and in sterile water following the manufacturer's instructions for in vitro and in vivo studies, respectively.
Broth dilution.
Antifungal susceptibility testing was performed by a broth microdilution method in accordance with the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) recommendations (8). The final concentrations of POS, CAS, and AMB ranged from 0.0078 to 8.0 μg/ml. The final concentrations of FLC ranged from 0.125 to 64 μg/ml. Plates were incubated at 35°C and read at 24 and 48 h. Readings were performed spectrophotometrically at an optical density at 490 nm with an automatic plate reader (ELx800; Biotek). POS, FLC, and CAS MICs were considered the first concentrations of the antifungal agents at which the turbidities in the wells were 50% less than those in the control wells (8, 9). The AMB MIC was considered the first concentration of the antifungal agent at which the turbidity in the well was 90% less than that in the control well (8). Experiments were performed in quintuplicate.
Killing curves.
Three to five colonies of C. glabrata isolates from a 48-h growth plate were suspended in 10 ml of sterile distilled water, and the turbidity was adjusted using spectrophotometric methods to an 0.5 McFarland standard (approximately 1 × 10 6 to 5 × 10 6 CFU/ml). One milliliter of the adjusted fungal suspension was added to 9 ml of either RPMI 1640 medium buffered with MOPS (morpholinepropanesulfonic acid) or a solution of growth medium plus an appropriate amount of drug. Drugs were used at concentrations of one-half and eight times the MICs. Test solutions were placed on a shaker and incubated at 35°C. At time points of 0, 2, 4, 8, and 24 h following the introduction of the test isolate into the system, 100-μl aliquots were removed from each test solution. After 10-fold serial dilutions, a 50-μl aliquot from each dilution was streaked in triplicate onto Sabouraud dextrose agar plates for colony count determination. Following incubation at 35°C for 48 h, the number of CFU on each plate was determined. The limit of detection was 20 CFU/ml. Fungicidal activity was considered to be achieved when the number of CFU per milliliter was <99.9% of the initial inoculum size (15). Each isolate was tested at least twice.
Animal experiments.
CD1 male mice (Charles River, Calco, Lecco, Italy) weighing 25 g were rendered neutropenic by intraperitoneal (i.p.) administration of cyclophosphamide (200 mg/kg of body weight) on days −4, +1, and +4 postinfection. They were infected intravenously (i.v.) through the lateral tail vein with C. glabrata 4198, 4205, and 4293 given in an 0.2-ml volume. A total of four studies were performed: in studies 1 and 2, the mice were challenged, respectively, with 2.44 × 108 CFU/mouse and 1.04 × 108 CFU/mouse of the FLC-dose-dependent-susceptible (SDD) isolate C. glabrata 4293; in study 3, they were challenged with 2.6 × 107 CFU/mouse of the FLC-susceptible (S) isolate 4205; in study 4, they were challenged with 5.8 × 107 CFU/mouse of the FLC-resistant (R) isolate 4198. Drugs were initiated 24 h postchallenge and given daily for six consecutive days. FLC was given in an 0.2-ml volume i.p. at doses ranging from 25 to 50 mg/kg/day. POS was administered by oral gavage in an 0.2-ml volume at doses of 15 and 30 mg/kg/day. CAS and AMB were administered i.p. at a dose of 1 mg/kg/day only in studies 1 and 2. Drug efficacy was assessed by determining the number of CFU per kidney pair. Briefly, the mice were sacrificed and the kidneys were homogenized. Diluted and undiluted aliquots, including the entire organ, were grown on Sabouraud dextrose agar for colony count determination. Tissue burden experiments were performed on day 7 postinfection. There were from seven to eight animals in each control and treatment group. Animal experiments were conducted with the approval of the University of Ancona Ethics Committee.
Statistical analysis.
The in vitro susceptibility data were compared by Student's t test or the Mann-Whitney U test. The Mann-Whitney U test was also used to compare tissue burden counts. A P value of <0.05 was considered statistically significant.
RESULTS
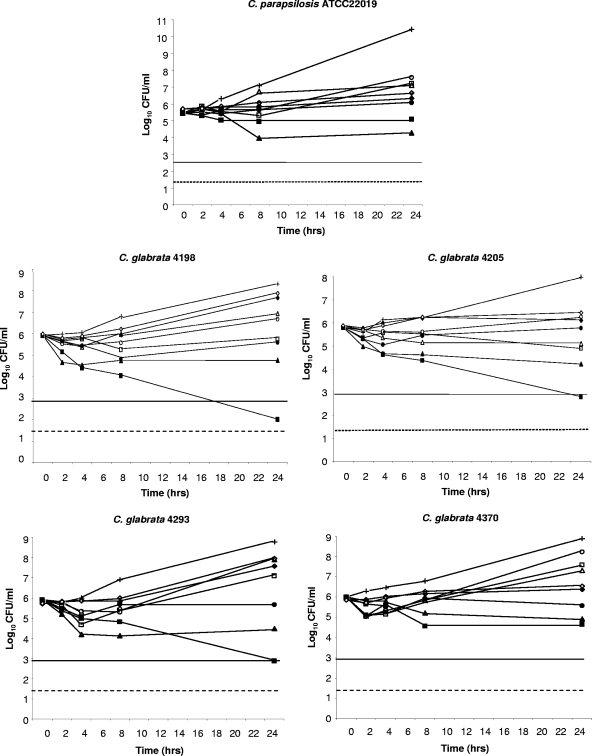
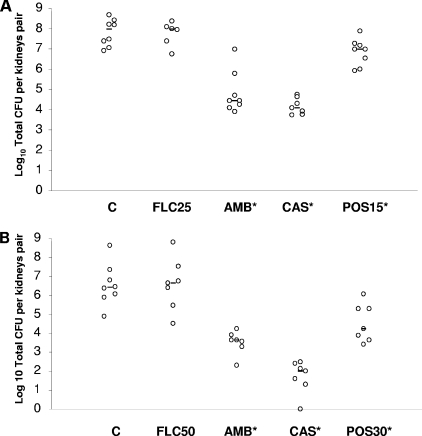
In vitro susceptibility testing results are reported in Table 1. Median FLC and POS MICs for C. parapsilosis ATCC 22019 were 4.0 and ≤0.03 μg/ml, respectively. Median CAS and AMB MICs for the quality control isolate were 0.25 and 1.0 μg/ml, respectively. FLC MICs ranged from 1.0 to >64.0 μg/ml, with C. glabrata 4205 being susceptible to the triazole and isolates 4370 and 4293 being susceptible in a dose-dependent manner, while isolate 4198 was resistant to this drug. POS MICs ranged from ≤0.03 to 0.5 μg/ml. AMB MICs ranged from 0.25 to 2.0 μg/ml. CAS MICs ranged from ≤0.03 to 0.25 μg/ml. The FLC MIC was significantly higher than those observed for POS (P < 0.001), CAS (P < 0.001), and AMB (P < 0.001). In general, when FLC MICs increased, so did POS MICs. Thus, the rank order of susceptibility to both azoles was 4205 (S) > 4370 (S) > 4293 (SDD) > 4198 (R) (P ranging from 0.008 to 0.03). Results of killing experiments are shown in Fig. 1. AMB at eight times the MIC was fungicidal against three isolates of C. glabrata (4198, 4205, and 4293). The same concentration of the polyene exerted a fungistatic activity against C. parapsilosis ATCC 22019 and C. glabrata 4370. AMB at one-half the MIC was fungistatic against all isolates. Similarly, CAS at one-half the MIC was shown to be fungistatic against all isolates. Although CAS at eight times the MIC was not fungicidal against any tested isolate, the echinocandin yielded a reduction of CFU/ml ranging from 1.08 to 1.58 log10 upon 24 h of incubation. POS and FLC exerted a fungistatic activity against all isolates at both one-half and eight times the MICs. Then, we investigated the activity of POS in an experimental model of disseminated candidiasis due to three different isolates of C. glabrata. Figure 2 shows the data from the experiments performed with the SDD strain 4293 (median FLC MIC, 32 μg/ml; range, 32 to 64 μg/ml). POS was given by oral gavage at 15 and 30 mg/kg/day in studies 1 and 2, respectively. As expected either AMB (P = 0.003) or CAS (P = 0.003) given at 1 mg/kg/day was effective at reducing the fungal burden against the controls. This was seen in study 1 and confirmed in study 2. Similarly, POS given at 15 mg/kg/day (P = 0.029) and at 30 mg/kg/day (P = 0.0037) significantly reduced the kidney counts below the control values. FLC was not effective at either 25 or 50 mg/kg/day.
TABLE 1.
In vitro susceptibilities of yeast isolates used in this study to FLC, POS, AMB, and CASa
| Isolateb | Median MIC (range) (μg/ml) of drug:
|
|||
|---|---|---|---|---|
| FLC | POS | AMB | CAS | |
| C. parapsilosis ATCC 22019 | 4.0 (2.0-4.0) | ≤0.03 (≤0.03) | 1.0 (1.0) | 0.25 (0.25) |
| C. glabrata 4198 | >64 (>64) | 0.5 (0.5) | 0.5 (0.5-1.0) | 0.25 (0.06-0.25) |
| C. glabrata 4205 | 1.0 (1.0-4.0) | ≤0.03 (≤0.03) | 0.5 (0.25-0.5) | 0.25 (0.25) |
| C. glabrata 4293 | 32 (32-64) | 0.25 (0.125-0.25) | 0.5 (0.5) | ≤0.03 (≤0.03-0.06) |
| C. glabrata 4370 | 16 (8.0-16) | 0.06 (0.06-0.125) | 1.0 (1.0-2.0) | 0.125 (0.125) |
Readings were performed spectrophotometrically at either 24 or 48 h with an automatic plate reader (ELx800; Biotek) set at 490 nm. Results were similar (within 1 double dilution). MICs are taken from 48-h readings for POS, FLC, and AMB and from 24-h readings for CAS (9).
Each isolate was tested five times.
FIG. 1.
Time-kill studies conducted with C. parapsilosis ATCC 22019 and four isolates of C. glabrata (4198, 4205, 4293, and 4370). Symbols: crosses, controls; open symbols, one-half the MIC of the drug; solid symbols, eight times the MIC of the drug; squares, AMB; triangles, CAS; circles, POS; diamonds, FLC. The solid lines represent a >99.9% growth reduction compared with the initial inoculum size (fungicidal effect). The limit of detection is 20 CFU/ml (dotted lines).
FIG. 2.
Kidney tissue burden of neutropenic CD1 mice infected i.v. with C. glabrata 4293. In study 1, the mice were challenged i.v. with 2.44 × 108 CFU/mouse (A), while in study 2 they were challenged i.v. with 1.04 × 108 CFU/mouse (B). Animals were treated daily for six consecutive days with CAS and AMB i.p. at 1 mg/kg/day in both studies, and FLC was given i.p. at 25 and 50 mg/kg/day in studies 1 and 2, respectively, while POS was given by oral gavage at 15 and 30 mg/kg/day in studies 1 and 2, respectively. Tissue burden experiments were performed on day 7 postinfection. C, control mice. The bars represent the medians. The asterisks indicate P values of <0.05.
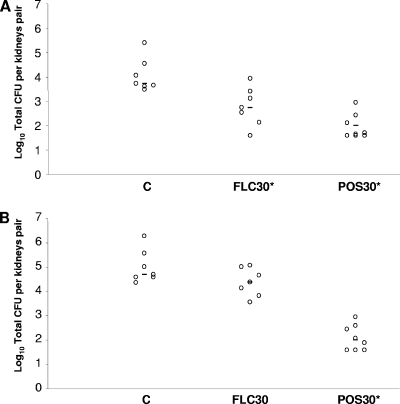
Figure 3A shows the in vivo results of the animals challenged with the S isolate 4205. Both triazole FLC and triazole POS given at 30 mg/kg/day were active at reducing the fungal burden with respect to the controls (P = 0.0070 and 0.0003, respectively). Finally, POS given at a dose of 30 mg/kg/day (P = 0.0003), but not FLC, reduced the colony count below that of the controls in the mice infected with the FLC-R strain (Fig. 3B).
FIG. 3.
Kidney tissue burden of neutropenic CD1 mice infected i.v. with the FLC-susceptible strain C. glabrata 4205 (A) and with the FLC-resistant strain C. glabrata 4198 (B). The mice were challenged i.v. with 2.6 × 107 CFU/mouse of isolate 4205 and with 5.8 × 107 CFU/mouse of isolate 4198. Animals were treated daily for six consecutive days with FLC i.p. at 30 mg/kg/day, while POS was given by oral gavage at 30/mg/kg/day. Tissue burden experiments were performed on day 7 postinfection. C, control mice. The bars represent the medians. The asterisks indicate P values of <0.05.
DISCUSSION
In this study, we investigated the in vitro and in vivo activities of POS against C. glabrata. Our susceptibility data agree with those previously reported by others who have found a slight increase in POS MICs as FLC MICs increased (13). However, our FLC-R isolate retained a relatively low POS MIC (0.5 μg/ml), representing an unusual condition with respect to that reported by recent reports (14, 16). In fact, Sabatelli et al. showed that among 149 FLC-R isolates of C. glabrata, POS MIC50 and MIC90 were 2.0 and 16 μg/ml, respectively (16). Similarly, Pfaller et al. showed that among 145 FLC-R isolates of C. glabrata, POS MIC50 and MIC90 were 8.0 and >8.0 μg/ml, respectively (14). Our data suggest that the phenotype of FLC-R C. glabrata used in our study is unusual and not representative of the more common pan-azole-resistant profile.
Recent clinical observations suggest that C. glabrata exhibits considerable clinically significant cross-resistance between older azole drugs (FLC and itraconazole) and voriconazole (VRC) (10, 11). According to these data, Panackal et al. state that caution is advised when considering VRC therapy for C. glabrata candidemia occurring in patients with extensive prior azole drug exposure (10). The clinical impact of POS cross-resistance is still poorly investigated. From a therapeutic point of view, POS appears to offer advantages over FLC and VRC, since the activity of the former triazole appears to be less affected by either mutations in ERG11 or the overexpression of specific efflux pumps (4). In recent studies, POS was shown to be effective for the treatment of oropharyngeal and esophageal candidiasis in subjects with human immunodeficiency virus infection including those who were azole refractory (17, 21). Very few data are still available on the efficacy of POS in infections due to C. glabrata. Anstead et al. reported a systemic infection due to C. glabrata that has been controlled by the use of POS after failing FLC (1). Overall, these data would indicate a beneficial effect of POS in clinical circumstances in which other triazoles failed. Cacciapuoti et al. tested POS in a murine model of systemic candidiasis due to C. albicans with variable patterns of FLC susceptibility (2). They showed that the new triazole was significantly efficacious against FLC-S, FLC-SDD, or FLC-R strains in either immunocompetent or immunocompromised mouse models. They showed that POS, but not FLC, was active against two FLC-R strains, including one with a POS MIC of 16 μg/ml, although they observed an activity reduction with respect to all the other strains (2).
Here, we demonstrated that POS is also active in vivo against all three strains of C. glabrata with different susceptibilities to FLC. This triazole was active against only one strain susceptible to FLC (4205; MICs ranging from 1.0 to 4.0 μg/ml), not against the other two strains of C. glabrata tested. Both AMB and CAS were included in studies 1 and 2 since these drugs represent common therapies for treating systemic infection due to C. glabrata (11). Our time-kill experiments showed that FLC, POS, and CAS were fungistatic against all isolates, while AMB at eight times the MIC was fungicidal against three out of four isolates of C. glabrata tested. Literature data reported so far showed that POS was fungistatic in the time-kill tests against all C. glabrata and C. parapsilosis strains, even at drug concentrations of 32 to 64 times the MIC (18). Moreover, the fungicidal activity of AMB against C. albicans is very fast, while for both C. glabrata and C. parapsilosis, the fungicidal endpoint is generally reached upon 24 h of incubation (3). Similar to these data, we showed that AMB reached a fungicidal activity starting from 17 h (C. glabrata 4198) to 24 h (C. glabrata 4205 and 4293) of incubation. Overall, these results underline the difficulties encountered during the treatment of invasive infections due to C. glabrata.
In conclusion, our data suggest that POS may be a useful option in the management of systemic infections caused by C. glabrata. Additionally, the new triazole may be a therapeutic choice in those cases where an FLC-R isolate is found to retain a relatively low POS MIC.
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Anstead, G. M., M. Martinez, and J. R. Graybill. 2006. Control of a Candida glabrata prosthetic endovascular infection with posaconazole. Med. Mycol. 44:273-277. [DOI] [PubMed] [Google Scholar]
- 2.Cacciapuoti, A., D. Loebenberg, E. Corcoran, F. Menzel, E. L. Moss, C. Norris, M. Michalski, K. Raynor, J. Halpern, C. Mendrick, B. Arnold, B. Antonacci, R. Parmegiani, T. Yarosh-Tomaine, G. H. Miller, and R. S. Hare. 2000. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob. Agents Chemother. 44:2017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canton, E., J. Peman, M. Gobernado, A. Viudes, and A. Espinel-Ingroff. 2004. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob. Agents Chemother. 48:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau, A. S., C. A. Mendrick, F. J. Sabatelli, D. Loebenberg, and P. M. McNicholas. 2004. Application of real-time quantitative PCR to molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob. Agents Chemother. 48:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornely, O. A., J. Maertens, D. J. Winston, J. Perfect, A. J. Ullmann, T. J. Walsh, D. Helfgott, J. Holowiecki, D. Stockelberg, Y. T. Goh, M. Petrini, C. Hardalo, R. Suresh, and D. Angulo-Gonzalez. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348-359. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg, R. N., K. Mullane, J. A. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta, S., N. G. Almyroudis, M. Battiwalla, B. J. Bambach, P. L. McCarthy, A. D. Proefrock, D. Ball, P. Paplham, A. Varma, J. Kwon-Chung, and B. H. Segal. 2007. Successful treatment of disseminated fusariosis with posaconazole during neutropenia and subsequent allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 9:156-160. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 9.Odds, F., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panackal, A. A., J. L. Gribskov, J. F. Staab, K. A. Kirby, M. Rinaldi, and K. A. Marr. 2006. Clinical significance of azole antifungal drug cross-resistance in Candida glabrata. J. Clin. Microbiol. 44:1740-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2008. Selection of a surrogate agent (fluconazole or voriconazole) for initial susceptibility testing of posaconazole against Candida spp.: results from a global antifungal surveillance program. J. Clin. Microbiol. 46:551-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skiest, D. J., J. A. Vazquez, G. M. Anstead, J. R. Graybill, J. Reynes, D. Ward, R. Hare, N. Boparai, and R. Isaacs. 2007. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin. Infect. Dis. 44:607-614. [DOI] [PubMed] [Google Scholar]
- 18.Sóczó, G., G. Kardos, P. M. McNicholas, E. Balogh, L. Gergely, I. Varga, B. Kelentey, and L. Majoros. 2007. Correlation of posaconazole minimum fungicidal concentration and time kill test against nine Candida species. J. Antimicrob. Chemother. 60:1004-1009. [DOI] [PubMed] [Google Scholar]
- 19.Torres, H. A., R. Y. Hachem, R. F. Chemaly, D. P. Kontoyiannis, and I. I. Raad. 2005. Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect. Dis. 5:775-785. [DOI] [PubMed] [Google Scholar]
- 20.Ullmann, A. J., J. H. Lipton, D. H. Vesole, P. Chandrasekar, A. Langston, S. R. Tarantolo, H. Greinix, W. Morais de Azevedo, V. Reddy, N. Boparai, L. Pedicone, H. Patino, and S. Durrant. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335-347. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez, J. A., D. J. Skiest, L. Nieto, R. Northland, I. Sanne, J. Gogate, W. Greaves, and R. Isaacs. 2006. A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS. Clin. Infect. Dis. 42:1179-1186. [DOI] [PubMed] [Google Scholar]
- 22.Walsh, T. J., I. Raad, T. F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R. Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal, D. A. Stevens, J. A. van Burik, C. S. White, G. Corcoran, J. Gogate, G. Krishna, L. Pedicone, C. Hardalo, and J. R. Perfect. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]