Abstract
A microdilution method was used to test 11 antifungal drugs against clinical isolates of Fusarium thapsinum and three different phylogenetic clades of Fusarium verticillioides that were characterized by sequencing a region of the β-tubulin gene. Terbinafine was the most-active drug against both species, followed by posaconazole against F. verticillioides.
Fusarium verticillioides (F. moniliforme) is one of the most-common species involved in fusariosis (7). These infections are frequently refractory to treatment because species of Fusarium are generally resistant to the currently available antifungal agents (1, 12). The information available on clinical infections by F. verticillioides is limited because in most cases of fusariosis, the identification of the causative agent is not performed, due to the difficulties in species recognition. F. verticillioides can be morphologically confused with other species of the Gibberella fujikuroi species complex (11, 14, 15). The purposes of this study have been (i) to verify molecularly the morphological identification of numerous clinical isolates of F. verticillioides, (ii) to determine whether they constitute a unique phylogenetic group, and in the case that different genetic groups were detected, (iii) to determine if they demonstrate various antifungal susceptibility patterns.
For these first two aims we have sequenced a region of the β-tubulin gene which has proven to be highly informative at the phylogenetic level in different molecular studies of the G. fujikuroi complex (13, 14, 15). In this phylogenetic study, we included a total of 46 strains, mainly from clinical sources, that have been morphologically identified as F. verticillioides (3). Twelve sequences retrieved from GenBank were also included, 10 of them corresponding to related species of the complex other than F. verticillioides and Fusarium thapsinum (14, 15) (Table 1). The procedures for DNA extraction and amplification and sequencing of the region analyzed have been previously described (5). With the primers used, TUB-F and T22 (2, 13), we were able to amplify and sequence a fragment of 433 bp. Surprisingly, a BLAST search demonstrated that four of the isolates did not belong to F. verticillioides; instead, they were identified as F. thapsinum. The morphological differentiation of F. thapsinum and F. verticillioides is problematic. According to Klittich et al. (8), the production of a yellow diffusible pigment on potato dextrose agar is the main phenotypic feature distinguishing the two species, but this pigment is not produced by all of the strains.
TABLE 1.
Isolates included in the study and their origina
| Species | Isolate no. | Isolate source | GenBank TUB |
|---|---|---|---|
| F. verticillioides | CBS 576.78 (T) | Clinical source, USSR | AM933108 |
| F. verticillioides | CBS 102699 | Clinical source, Germany | AM933097 |
| F. verticillioides | CBS 108922 | Clinical source, Germany | AM933102 |
| F. verticillioides | CBS 115135 | Clinical source, Sweden | AM933089 |
| F. verticillioides | FMR 7236 | Clinical source, Spain | AM933098 |
| F. verticillioides | FMR 8585 | Clinical source, Spain | AM933094 |
| F. verticillioides | FMR 8694 | Clinical source, Spain | AM933111 |
| F. verticillioides | UTHSC R-1027 | Clinical source, United States | AM933092 |
| F. verticillioides | UTHSC R-1213 | Clinical source, United States | AM933112 |
| F. verticillioides | UTHSC R-1214 | Clinical source, United States | AM933115 |
| F. verticillioides | UTHSC 90-715 | Clinical source, United States | AM933118 |
| F. verticillioides | UTHSC 93-459 | Clinical source, United States | AM933116 |
| F. verticillioides | UTHSC 94-106 | Clinical source, United States | AM933105 |
| F. verticillioides | UTHSC 95-2483 | Clinical source, United States | AM933099 |
| F. verticillioides | UTHSC 96-7 | Clinical source, United States | AM933110 |
| F. verticillioides | UTHSC 96-449 | Clinical source, United States | AM933101 |
| F. verticillioides | UTHSC 96-2334 | Clinical source, United States | AM933113 |
| F. verticillioides | UTHSC 99-1013 | Clinical source, United States | AM932522 |
| F. verticillioides | UTHSC 99-1936 | Clinical source, United States | AM933109 |
| F. verticillioides | UTHSC 00-1810 | Clinical source, United States | AM933119 |
| F. verticillioides | UTHSC 02-185 | Clinical source, United States | AM933122 |
| F. verticillioides | UTHSC 03-72 | Clinical source, United States | AM933114 |
| F. verticillioides | UTHSC 03-504 | Clinical source, United States | AM933100 |
| F. verticillioides | UTHSC 03-1454 | Clinical source, United States | AM933103 |
| F. verticillioides | UTHSC 03-1455 | Clinical source, United States | AM933104 |
| F. verticillioides | UTHSC 03-2552 | Clinical source, United States | AM933106 |
| F. verticillioides | UTHSC 04-506 | Clinical source, United States | AM933130 |
| F. verticillioides | UTHSC 04-695 | Clinical source, United States | AM933132 |
| F. verticillioides | UTHSC 05-430 | Clinical source, United States | AM933131 |
| F. verticillioides | UTHSC 05-431 | Clinical source, United States | AM932521 |
| F. verticillioides | UTHSC 05-1039 | Clinical source, United States | AM933090 |
| F. verticillioides | UTHSC 05-3141 | Clinical source, United States | AM933091 |
| F. verticillioides | UTHSC 06-134 | Clinical source, United States | AM933121 |
| F. verticillioides | UTHSC 06-1103 | Clinical source, United States | AM933128 |
| F. verticillioides | UTHSC 06-1639 | Clinical source, United States | AM933129 |
| F. verticillioides | UTHSC 06-3023 | Clinical source, United States | AM933120 |
| F. verticillioides | CBS 139.40 | Phyllocactus hybridus, Italy | AM933107 |
| F. verticillioides | FMR 9323 | Corn, Spain | AM933117 |
| F. verticillioides | FMR 9324 | Pig feed, Spain | AM933093 |
| F. verticillioides | FMR 9325 | Horse feed, Spain | AM933096 |
| F. verticillioides | FMR 8976 | Unknown | AM933095 |
| F. verticillioides | U34413b | ||
| F. thapsinumc | CBS 539.79 | Clinical source, Italy | AM933124 |
| F. thapsinumc | UTHSC 98-1202 | Clinical source, United States | AM933126 |
| F. thapsinumc | UTHSC 03-2158 | Clinical source, United States | AM933125 |
| F. thapsinumc | UTHSC 03-3093 | Clinical source, United States | AM933123 |
| F. thapsinum | CBS 733.97 | Sorghum bicolor, South Africa | AM933127 |
| F. thapsinum | U34418b | ||
| F. denticulatum | U61550b | ||
| F. fujikuroi | U34415b | ||
| F. lactis | U61551b | ||
| F. napiforme | U34428b | ||
| F. nygamai | U34426b | ||
| F. pseudoanthophilum | U61553b | ||
| F. pseudocircinatum | U34427b | ||
| F. ramigenum | U61554b | ||
| F. sacchari | U34414b | ||
| F. subglutinans | U34417b |
TUB, β-tubulin gene; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; FMR, Facultat de Medicina i Ciències de la Salut, Reus, Spain; UTHSC, University of Texas Health Science Center at San Antonio, San Antonio, TX; (T), type strain.
Sequences retrieved from GenBank.
Isolates morphologically identified as F. verticillioides.
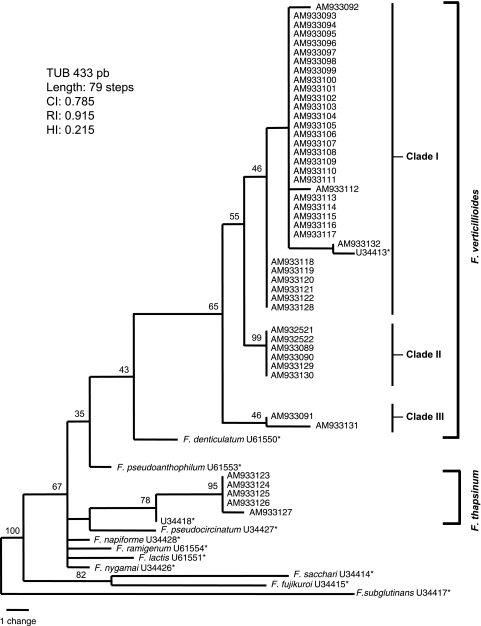
Parsimony analysis of the data set yielded 120 phylogenetic trees of 79 steps in length (Fig. 1). F. verticillioides and F. thapsinum were clearly separated from the other species; however, F. verticillioides showed a high molecular variability, which was reflected in the existence of three different molecular clades (I, II, and III) and nine different haplotypes. Whether these clades represented different reproductively isolated subgroups can only be determined by the analysis of additional, independent, variable sequence data sets.
FIG. 1.
One of the 120 most-parsimonious trees obtained from heuristic searches based on β-tubulin gene (TUB) sequences. Bootstrap support values are indicated at the nodes. CI, consistency index; RI, retention index; HI, homoplasy index. Asterisks indicate accession numbers of sequences retrieved from GenBank.
We then evaluated the in vitro activity of 11 antifungal drugs against 5 isolates of F. thapsinum and 24 of F. verticillioides that were randomly selected from the different clades. The isolates were grown on potato dextrose agar plates and incubated at 25°C for 7 days. We used a microdilution reference method (10), with some modifications. The inocula were adjusted to a final concentration of 4 × 103 to 5 × 104 conidia/ml. Final drug concentrations ranged from 64 to 0.12 μg/ml for fluconazole and flucytosine, from 128 to 0.25 μg/ml for micafungin, and from 16 to 0.03 μg/ml for albaconazole, amphotericin B, itraconazole, ketoconazole, posaconazole, ravuconazole, terbinafine, and voriconazole. The MIC endpoint for amphotericin B, terbinafine, and most triazoles was defined as the lowest concentration that produced complete inhibition of growth; for fluconazole, flucytosine, ketoconazole, and micafungin, the endpoint was defined as the lowest concentration that produced 50% inhibition of growth. Testing was performed twice on two different days, and in those instances where the results did not coincide it was repeated a third time. For those strains, the MIC was considered as the mode of the three MICs.
The susceptibility results are shown in Table 2. For F. verticillioides, terbinafine was the most-active drug, followed by posaconazole, ravuconazole, voriconazole, amphotericin B, ketoconazole, albaconazole, and itraconazole in decreasing order of potency. Among these, itraconazole has practically no activity. For F. thapsinum, terbinafine was the most-active drug. Voriconazole and amphotericin B followed terbinafine with equivalent potencies. The rest of the tested drugs were not active against this species. In general, the differences among the MICs of the molecular clades, determined by using the Mann-Whitney U test (P < 0.05), were not statistically significant, with the exception of those for ketoconazole and ravuconazole, which showed less activity against the isolates of clades II and III than those of clade I. Although amphotericin B and voriconazole are the recommended drugs for treating fusariosis (4) and reasonable levels of clinical success (45.5%) have been attained with voriconazole (18), here both drugs showed more-limited activity than that of terbinafine for F. thapsinum and of terbinafine and posaconazole for F. verticillioides. Unlike F. verticillioides, posaconazole was not active against F. thapsinum. Fluconazole, flucytosine, and micafungin demonstrated no activity against any of the isolates tested, as had already been demonstrated (6, 19, 21). In a previous in vitro study, terbinafine combined with different azoles, such as albaconazole, ravuconazole, and voriconazole, showed synergistic activity against the three isolates of F. verticillioides that were tested (17). No data exists on the clinical use of terbinafine to treat infections by F. verticillioides. In some clinical trials, successful outcomes have been reported in patients with fusariosis treated with posaconazole, but the species involved in such cases were not determined (20).
TABLE 2.
Activities of conventional and new antifungal drugs against clinical isolates of F. verticillioides and F. thapsinuma
| Species and cladeb (no. of isolates tested) | MIC [μg/ml; range (GM)]
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ABC | AMB | ITC | KTC | PSC | RVC | TBF | VRC | |
| F. verticillioides | ||||||||
| Clade I (16) | 2-4 (3.03) | 2-4 (2.41) | 2->16 (12.70) | 1-4 (2.00) | 0.5-1 (0.79) | 1-4 (1.45) | 0.125-1 (0.21) | 2 (2.00) |
| Clade II (6) | 2-4 (3.56) | 2-4 (2.24) | >16 (>16) | 4->16 (8) | 0.5-1 (0.89) | 1-4 (2.24) | 0.125-1 (0.31) | 2-4 (2.83) |
| Clade III (2) | 4-8 (5.66) | 2 (2) | >16 (>16) | 4-16 (8) | 1 (1) | 4 (4) | 0.125-0.5 (0.25) | 2 (2) |
| Total (24) | 2-8 (3.34) | 2-4 (2.33) | 2->16 (17.51) | 1->16 (3.24) | 0.5-1 (0.83) | 1-4 (1.77) | 0.125-1 (0.24) | 2-4 (2.19) |
| F. thapsinum (5) | 16->16 (18.38) | 2-4 (2.64) | >16 (>16) | >16 (>16) | >16 (>16) | 8->16 (18.38) | 0.25-0.5 (0.44) | 2-4 (2.64) |
GM, geometric mean; ABC, albaconazole; AMB, amphotericin B; ITC, itraconazole; KTC, ketoconazole; PSC, posaconazole; RVC, ravuconazole; TBF, terbinafine; VRC, voriconazole.
See Fig. 1.
These results are very encouraging because, unlike other pathogenic species of Fusarium (1), at least two drugs, posaconazole and terbinafine, seem to exert some activity against F. verticillioides. This fact, together with the results shown in animal studies, where F. verticillioides was less virulent than Fusarium solani (9), would suggest a better prognosis for those infections caused by F. verticillioides than for those caused by F. solani.
This is the first in vitro study of the antifungal susceptibility of F. thapsinum. Although F. thapsinum is an important plant pathogen, several human infections have also been attributed to this species (16, 22). This study emphasizes the usefulness of molecular methods for the correct identification of species difficult to distinguish morphologically and has demonstrated important differences in the antifungal susceptibility patterns of F. verticillioides and F. thapsinum.
Acknowledgments
We thank Núria Pilas, Catalina Núñez, Marçal Mariné, M. Mar Rodríguez, Enrique Calvo, and Manuela Reyes for their contributions to this work.
This work was supported by the Spanish Ministerio de Ciencia y Tecnología, grants CGL2005-07394/BOS and CGL 2007-65669/BOS.
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Azor, M., J. Gené, J. Cano, and J. Guarro. 2006. Universal in vitro antifungal resistance of genetic clades of the Fusarium solani species complex. Antimicrob. Agents Chemother. 51:1500-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruse, M., R. Telerant, T. Gallagher, T. Lee, and J. W. Taylor. 2002. Cryptic species in Stachybotrys chartarum. Mycologia 94:814-822. [PubMed] [Google Scholar]
- 3.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 4.Dignani, M. C., and E. J. Anaissie. 2004. Human fusariosis. Clin. Microbiol. Infect. 1:67-75. [DOI] [PubMed] [Google Scholar]
- 5.Gilgado, F., J. Cano, J. Gené, and J. Guarro. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J. Clin. Microbiol. 43:4930-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groll, A. H., T. Stergiopoulou, E. Roilides, and T. J. Walsh. 2005. Micafungin: pharmacology, experimental therapeutics and clinical applications. Exp. Opin. Investig. Drugs 14:489-509. [DOI] [PubMed] [Google Scholar]
- 7.Guarro, J., and J. Gené. 1995. Opportunistic fusarial infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 14:741-754. [DOI] [PubMed] [Google Scholar]
- 8.Klittich, C., J. F. Leslie, P. E. Nelson, and W. F. Marasas. 1997. Fusarium thapsinum (Gibberella thapsina): a new species in section Liseola from sorghum. Mycologia 89:643-652. [Google Scholar]
- 9.Mayayo, E., I. Pujol, and J. Guarro. 1999. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J. Med. Microbiol. 48:363-366. [DOI] [PubMed] [Google Scholar]
- 10.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 11.Nirenberg, I., and K. O'Donnell. 1998. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 90:434-458. [Google Scholar]
- 12.Nucci, M., and E. Anaissie. 2007. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Donnell, K., and E. Cigelnik. 1997. Two divergent intragenomic rADN ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7:103-116. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell, K., E. Cigelnik, and H. I. Nirenberg. 1998. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465-493. [Google Scholar]
- 15.O'Donnell, K., H. I. Nirenberg, A. Takayuki, and E. Cigelnik. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41:61-78. [Google Scholar]
- 16.O'Donnell, K., B. A. J. Sarver, M. Brandt, D. C. Chang, J. Noble-Wang, B. J. Park, D. A. Sutton, L. Benjamin, M. Lindsley, A. Padhye, D. M. Geiser, and T. J. Ward. 2007. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 45:2235-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortoneda, M., J. Capilla, F. J. Pastor, I. Pujol, and J. Guarro. 2004. In vitro interactions of licensed and novel antifungal drugs against Fusarium spp. Diagn. Microbiol. Infect. Dis. 48:69-71. [DOI] [PubMed] [Google Scholar]
- 18.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nübling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 9:1122-1131. [DOI] [PubMed] [Google Scholar]
- 19.Pujol, I., J. Guarro, J. Gené, and J. Sala. 1997. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrob. Chemother. 39:163-167. [DOI] [PubMed] [Google Scholar]
- 20.Raad, I. I., R. Y. Hachem, R. Herbrecht, J. R. Graybill, R. Hare, G. Corcoran, and D. P. Kontoyiannis. 2006. Posaconazole as salvage of invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin. Infect. Dis. 42:1398-1403. [DOI] [PubMed] [Google Scholar]
- 21.Reuben, A., E. Anaissie, P. E. Nelson, R. Hashem, C. Legrand, D. H. Ho, and G. P. Bodey. 1989. Antifungal susceptibility of 44 clinical isolates of Fusarium species determined by using a microdilution method. Antimicrob. Agents Chemother. 33:1647-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yücesoy, M., M. C. Ergon, H. Oren, and Z. Gülay. 2004. Case report: a Fusarium fungemia. Mikrobiyol. Bul. 38:265-271. [PubMed] [Google Scholar]