Abstract
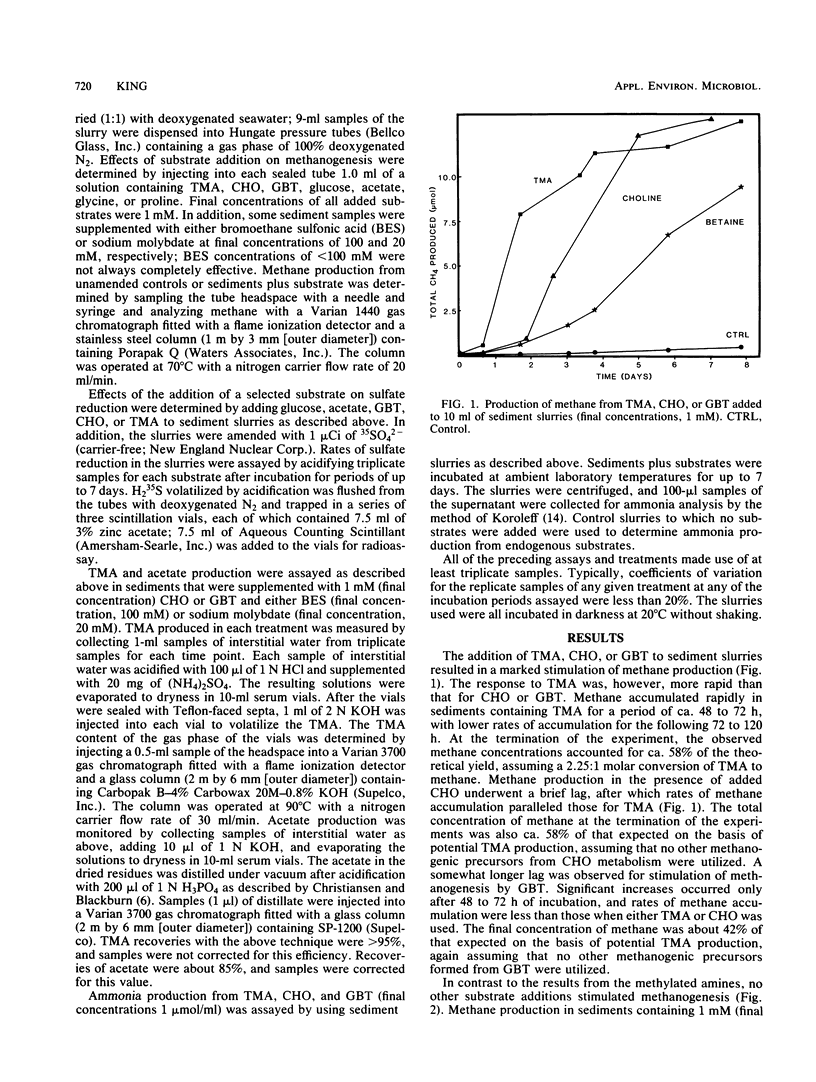
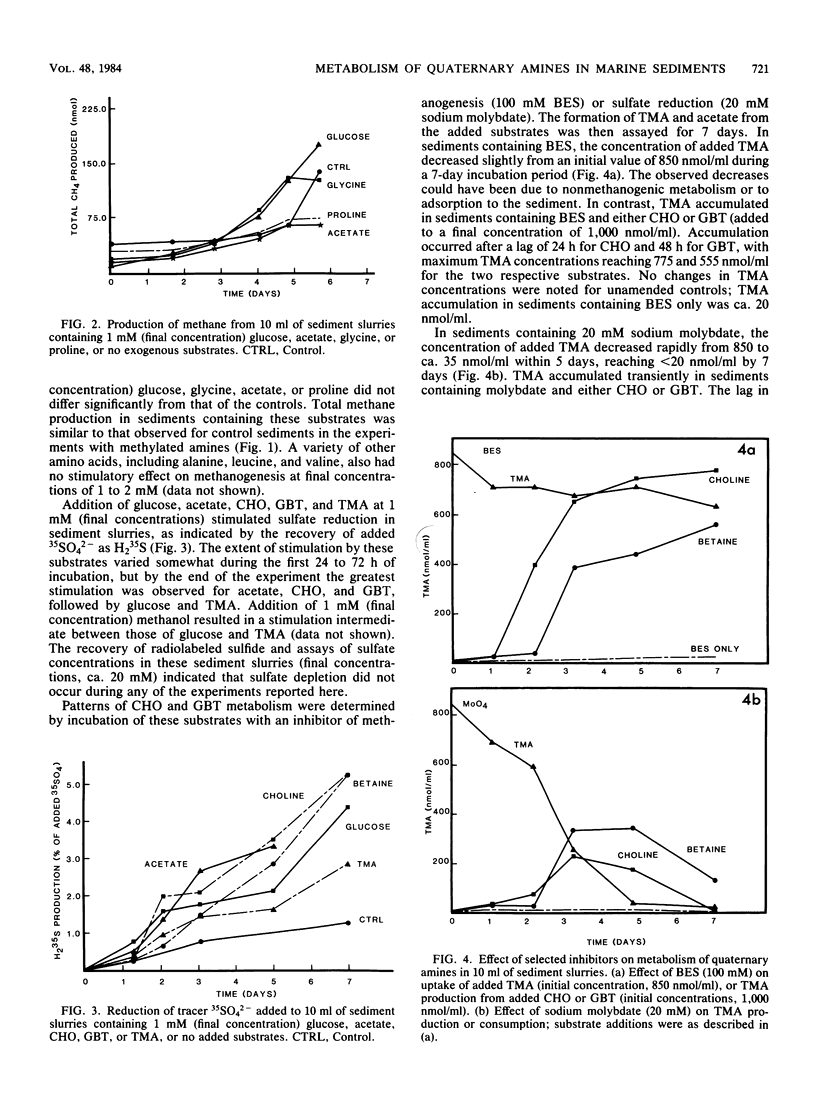
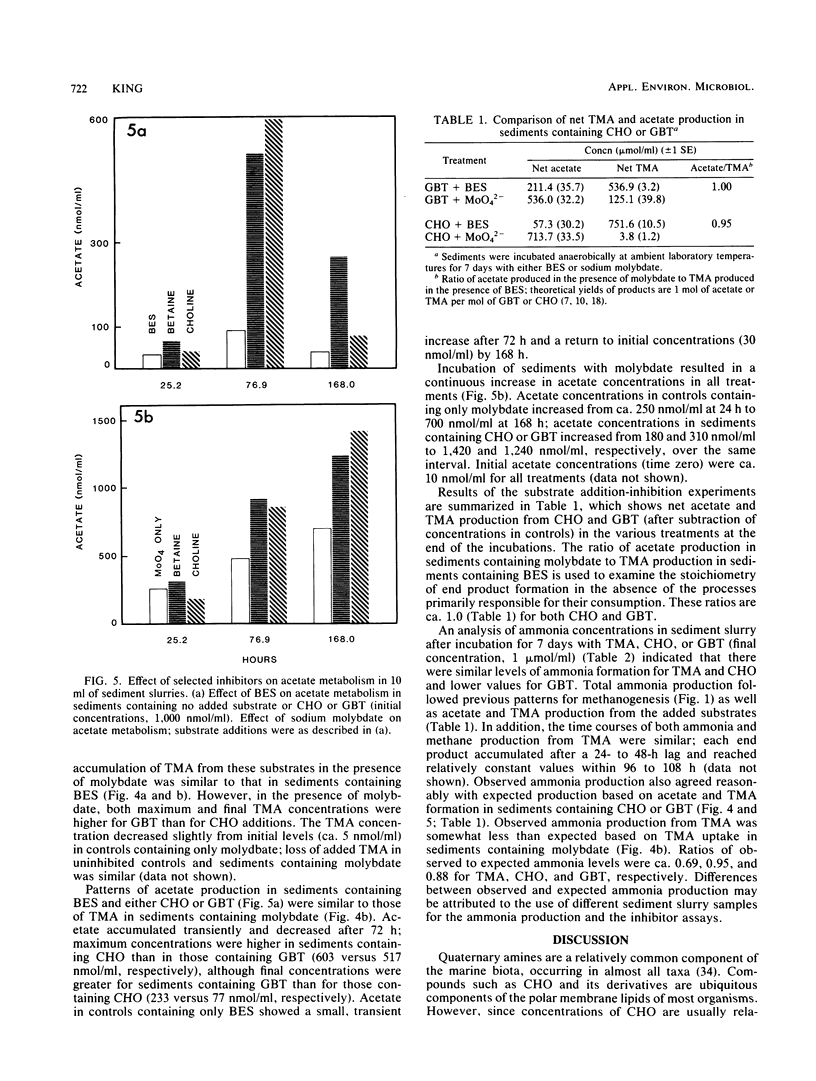
The response of methanogenesis and sulfate reduction to trimethylamine, choline, and glycine betaine was examined in surface sediments from the intertidal region of Lowes Cove, Maine. Addition of these substrates markedly stimulated methanogenesis in the presence of active sulfate reduction, whereas addition of other substrates, including glucose, acetate, and glycine, had no effect on methane production. Sulfate reduction was stimulated simultaneously with methanogenesis by the various quaternary amines and all other substrates examined. Incubation of exogenous trimethylamine, choline, or glycine betaine with either bromoethane sulfonic acid or sodium molybdate was used to establish pathways of degradation of the substrates. Methanogenesis dominated the metabolism of trimethylamine, although limited nonmethanogenic activity, perhaps by sulfate-reducing bacteria, was observed. Acetate was oxidized primarily by sulfate reducers. Both choline and glycine betaine were fermented stoichiometrically to acetate and trimethylamine; apparently, neither substrate could be utilized directly by methanogens or sulfate reducers, and the activities of fermenters, methanogens, and sulfate reducers were all required to effect complete mineralization. These observations support the hypothesis that the presence of quaternary amines can mediate the coexistence of sulfate reduction and methanogenesis in marine surface sediments; they also implicate methanogens in the nitrogen cycle of marine sediments containing quaternary amines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beers J. R. The species distribution of some naturally-occurring quaternary ammonium compounds. Comp Biochem Physiol. 1967 Apr;21(1):11–21. doi: 10.1016/0010-406x(67)90109-0. [DOI] [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. 1. Preparation and properties of cell-free extracts. J Biol Chem. 1965 Dec;240(12):4669–4674. [PubMed] [Google Scholar]
- Bricteux-Grégoire S., Duchâteau-Bosson G., Jeuniaux D., Florkin M. Constituants osmotiquement actifs des muscles adducteurs de mytilus edulis adaptée à l'eau de mer ou à l'eau saumâtre. Arch Int Physiol Biochim. 1964 Jan;72(1):116–123. doi: 10.3109/13813456409105257. [DOI] [PubMed] [Google Scholar]
- Fiebig K., Gottschalk G. Methanogenesis from Choline by a Coculture of Desulfovibrio sp. and Methanosarcina barkeri. Appl Environ Microbiol. 1983 Jan;45(1):161–168. doi: 10.1128/aem.45.1.161-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYWARD H. R., STADTMAN T. C. Anaerobic degradation of choline. I. Fermentation of choline by an anaerobic, cytochrome-producing bacterium, Vibrio cholinicus n. sp. J Bacteriol. 1959 Oct;78:557–561. doi: 10.1128/jb.78.4.557-561.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J., Lovley D. R. Metabolism of acetate, methanol, and methylated amines in intertidal sediments of lowes cove, maine. Appl Environ Microbiol. 1983 Jun;45(6):1848–1853. doi: 10.1128/aem.45.6.1848-1853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Wiebe W. J. Tracer analysis of methanogenesis in salt marsh soils. Appl Environ Microbiol. 1980 Apr;39(4):877–881. doi: 10.1128/aem.39.4.877-881.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983 Jul;46(1):152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A., Mays E. L., Tiedje J. M. Carbon and electron flow in mud and sandflat intertidal sediments at delaware inlet, nelson, new zealand. Appl Environ Microbiol. 1980 Apr;39(4):686–694. doi: 10.1128/aem.39.4.686-694.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E., Fahlbusch K., Walther R., Gottschalk G. Formation of N,N-Dimethylglycine, Acetic Acid, and Butyric Acid from Betaine by Eubacterium limosum. Appl Environ Microbiol. 1981 Sep;42(3):439–445. doi: 10.1128/aem.42.3.439-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann E., Hippe H., Gottschalk G. Betaine: New Oxidant in the Stickland Reaction and Methanogenesis from Betaine and l-Alanine by a Clostridium sporogenes-Methanosarcina barkeri Coculture. Appl Environ Microbiol. 1983 Feb;45(2):474–483. doi: 10.1128/aem.45.2.474-483.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill A. R., Grime D. W., Dawson R. M. Conversion of choline methyl groups through trimethylamine into methane in the rumen. Biochem J. 1978 Mar 15;170(3):529–535. doi: 10.1042/bj1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafaeli-Eshkol D., Avi-Dor Y. Studies on halotolerance in a moderately halophilic bacterium. Effect of betaine on salt resistance of the respiratory system. Biochem J. 1968 Oct;109(4):687–691. doi: 10.1042/bj1090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Ferry J. G. Isolation and Characterization of a Methylotrophic Marine Methanogen, Methanococcoides methylutens gen. nov., sp. nov. Appl Environ Microbiol. 1983 Feb;45(2):684–690. doi: 10.1128/aem.45.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Ward D. M. Substrates for sulfate reduction and methane production in intertidal sediments. Appl Environ Microbiol. 1983 Jan;45(1):193–199. doi: 10.1128/aem.45.1.193-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]



