Abstract
Heat treatment of Clostridium acetobutylicum SA-1 protoplasts at 55°C for 15 min before transformation resulted in expression in this microorganism of the kanamycin resistance determinant associated with plasmid pUB110. No heat treatment, or heat treatment at 65 or 44°C for various time intervals, resulted in no kanamycin resistance transformants being recovered on selective kanamycin-containing regeneration medium. DNase plate assay indicated that treatment at 55°C for 15 min completely inactivated the DNase activity associated with SA-1 protoplasts. Treatment of protoplasts at 65 or 55°C for various periods under simulated transformation conditions had an inhibitory effect, although prolonged treatment at 55 or 44°C appeared to stimulate DNase activity. Inactivation of protoplast-associated DNase activity by heat treatment at 55°C for 15 min correlated with successful expression of kanamycin resistance and suggests that an extremely active, heatsensitive, protoplast-associated DNase may be a factor in the polyethylene glycol-induced transformation of C. acetobutylicum SA-1 protoplasts. Plasmid pUB110 DNA was isolated from C. acetobutylicum SA-1 kanamycin-resistant (Kmr) transformant cultures by a modification of the procedure used for C. perfringens plasmids. Detection of pUB110 DNA was possible only when diethyl pyrocarbonate was incorporated into isolation protocols to inactivate DNase activity. Restriction studies further verified the presence of pUB110 DNA in C. acetobutylicum SA-1 Kmr transformants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcock E. R., Reid S. J., Jones D. T., Woods D. R. Clostridium acetobutylicum Protoplast Formation and Regeneration. Appl Environ Microbiol. 1982 Mar;43(3):719–721. doi: 10.1128/aem.43.3.719-721.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard K., Schrempf H., Goebel W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J Bacteriol. 1978 Feb;133(2):897–903. doi: 10.1128/jb.133.2.897-903.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
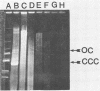
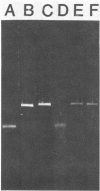
- Blaschek H. P., Klacik M. A. Role of DNase in recovery of plasmid DNA from Clostridium perfringens. Appl Environ Microbiol. 1984 Jul;48(1):178–181. doi: 10.1128/aem.48.1.178-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschek H. P., Solberg M. Isolation of a plasmid responsible for caseinase activity in Clostridium perfringens ATCC 3626B. J Bacteriol. 1981 Jul;147(1):262–266. doi: 10.1128/jb.147.1.262-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Ehrenberg L., Fedorcsak I., Solymosy F. Diethyl pyrocarbonate in nucleic acid research. Prog Nucleic Acid Res Mol Biol. 1976;16:189–262. doi: 10.1016/s0079-6603(08)60758-8. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T., Contente S., Dubnau D. Molecular cloning of heterologous chromosomal DNA by recombination between a plasmid vector and a homologous resident plasmid in Bacillus subtilis. Mol Gen Genet. 1980 Feb;177(3):459–467. doi: 10.1007/BF00271485. [DOI] [PubMed] [Google Scholar]
- Hammes W., Schleifer K. H., Kandler O. Mode of action of glycine on the biosynthesis of peptidoglycan. J Bacteriol. 1973 Nov;116(2):1029–1053. doi: 10.1128/jb.116.2.1029-1053.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic studies with bacterial protoplasts. Annu Rev Microbiol. 1981;35:237–272. doi: 10.1146/annurev.mi.35.100181.001321. [DOI] [PubMed] [Google Scholar]
- Jandová Z., Tichý P. Transformation of protoplasts of nontransformable Bacillus subtilis mutants by plasmid pUB 110 DNA. Folia Microbiol (Praha) 1982;27(6):465–467. doi: 10.1007/BF02876461. [DOI] [PubMed] [Google Scholar]
- Keggins K. M., Lovett P. S., Duvall E. J. Molecular cloning of genetically active fragments of Bacillus DNA in Bacillus subtilis and properties of the vector plasmid pUB110. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1423–1427. doi: 10.1073/pnas.75.3.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Blaschek H. P. Butanol Production by a Butanol-Tolerant Strain of Clostridium acetobutylicum in Extruded Corn Broth. Appl Environ Microbiol. 1983 Mar;45(3):966–973. doi: 10.1128/aem.45.3.966-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottes M., Grandi G., Sgaramella V., Canosi U., Morelli G., Trautner T. A. Different specific activities of the monomeric and oligomeric forms of plasmid DNA in transformation of B. subtilis and E. coli. Mol Gen Genet. 1979 Jul 24;174(3):281–286. doi: 10.1007/BF00267800. [DOI] [PubMed] [Google Scholar]
- Nestle M., Roberts W. K. An extracellular nuclease from Serratia marcescens. I. Purification and some properties of the enzyme. J Biol Chem. 1969 Oct 10;244(19):5213–5218. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Reid J. D., Stoufer S. D., Ogrydziak D. M. Efficient transformation of Serratia marcescens with pBR322 plasmid DNA. Gene. 1982 Jan;17(1):107–112. doi: 10.1016/0378-1119(82)90106-8. [DOI] [PubMed] [Google Scholar]
- Reid S. J., Allcock E. R., Jones D. T., Woods D. R. Transformation of Clostridium acetobutylicum Protoplasts with Bacteriophage DNA. Appl Environ Microbiol. 1983 Jan;45(1):305–307. doi: 10.1128/aem.45.1.305-307.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald M., Bouanchaud D., Bieth G., Prévot A. R. Nature plasmidique de la résistance à plusieurs antibiotiques chez C. perfringens type A, souche 659. C R Acad Sci Hebd Seances Acad Sci D. 1975 May 26;280(20):2401–2404. [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Plasmid replication in DNA Ts mutants of Bacillus subtilis. Plasmid. 1978 Jun;1(3):405–416. doi: 10.1016/0147-619x(78)90055-0. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Van Der Westhuizen A., Jones D. T., Woods D. R. Autolytic Activity and Butanol Tolerance of Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Dec;44(6):1277–1281. doi: 10.1128/aem.44.6.1277-1281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Yeggy J. P., Markovetz A. J. Role of nucleases in the isolation of plasmid deoxyribonucleic acid from Pseudomonas cepacia 4G9. J Bacteriol. 1980 Aug;143(2):1057–1059. doi: 10.1128/jb.143.2.1057-1059.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]