Abstract
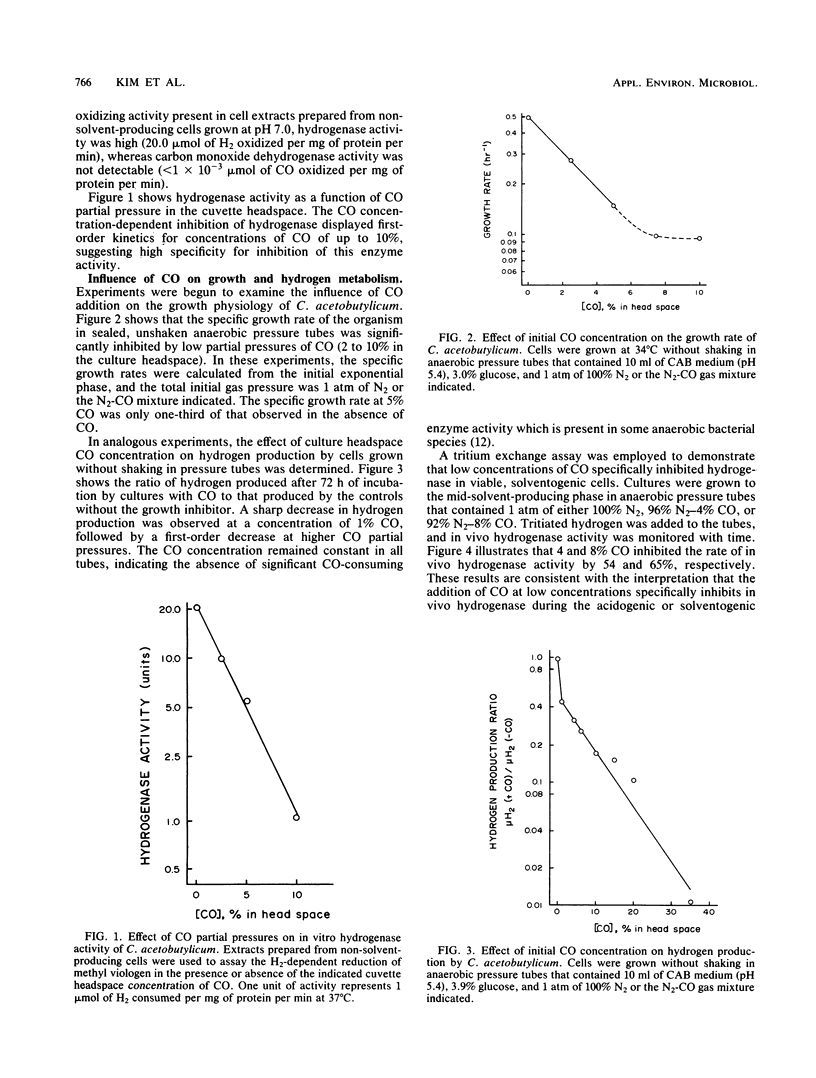
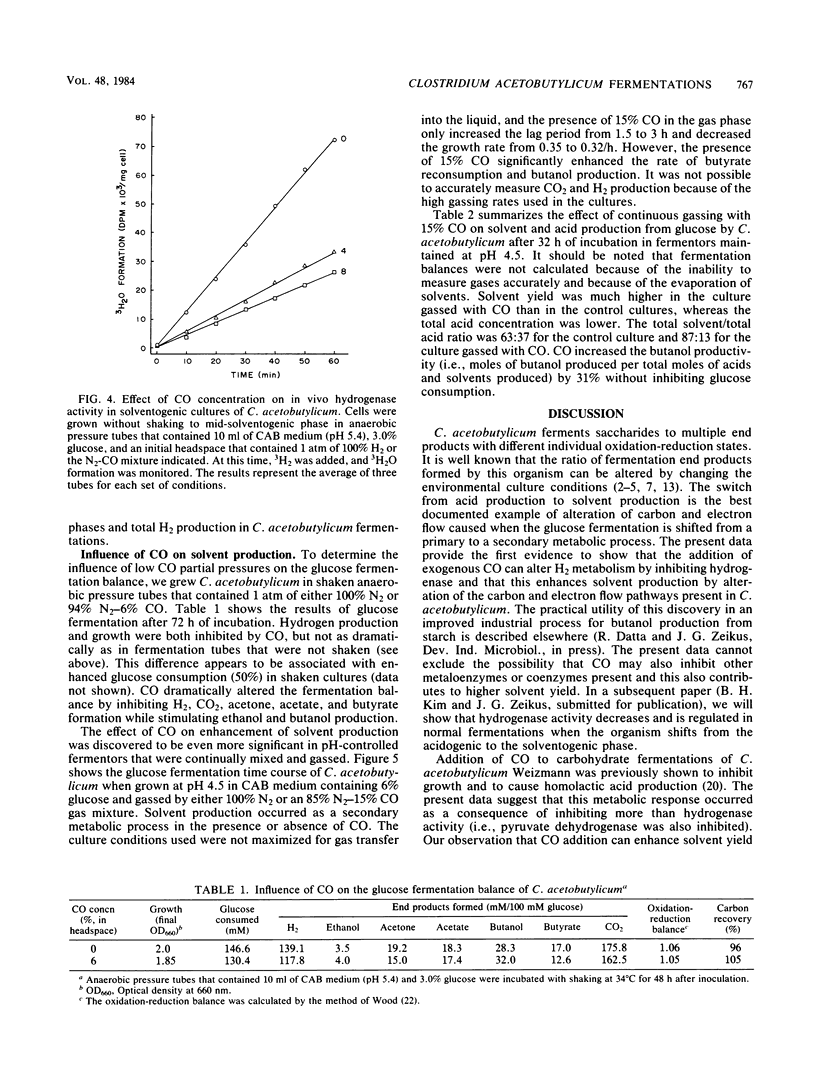
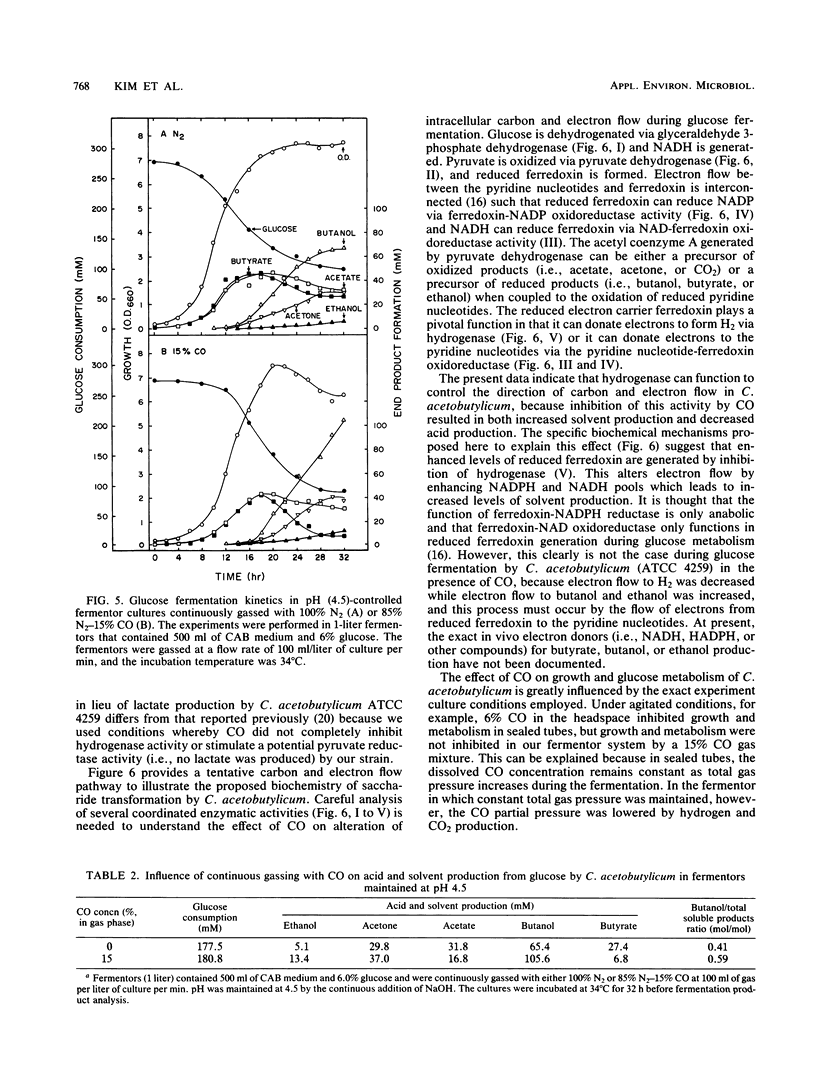
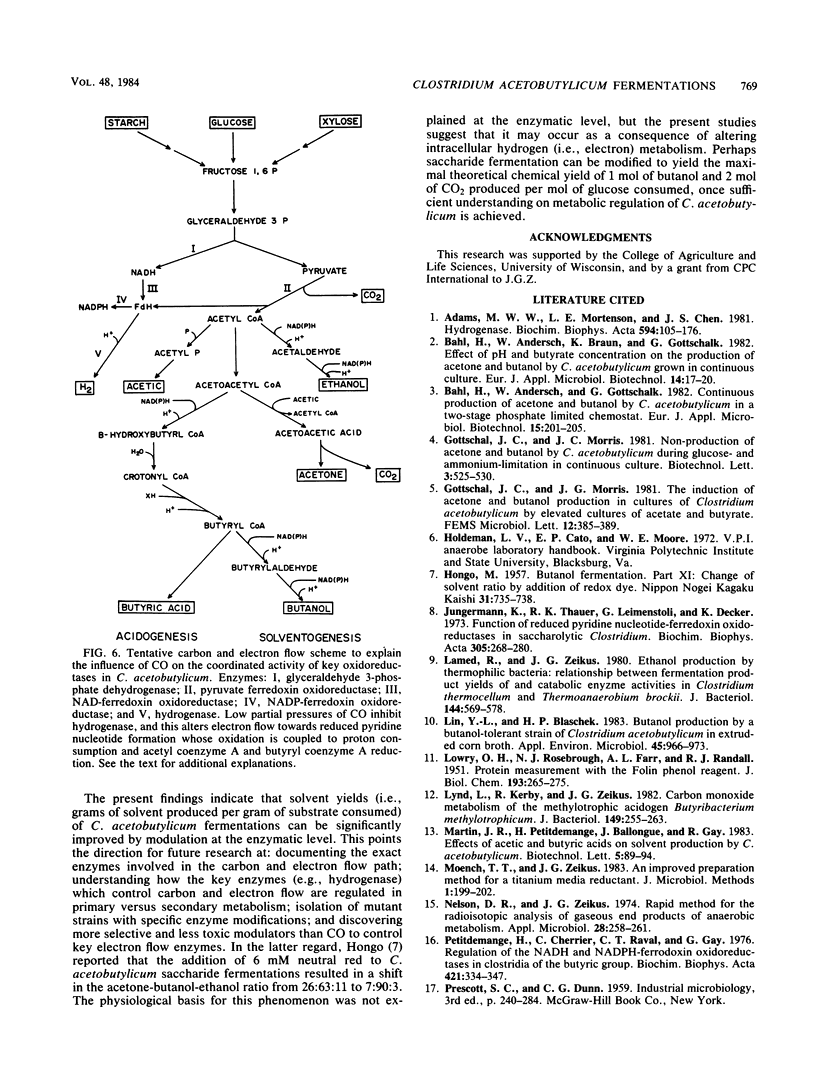
Extracts prepared from non-solvent-producing cells of Clostridium acetobutylicum contained methyl viologen-linked hydrogenase activity (20 U/mg of protein at 37°C) but did not display carbon monoxide dehydrogenase activity. CO addition readily inhibited the hydrogenase activity of cell extracts or of viable metabolizing cells. Increasing the partial pressure of CO (2 to 10%) in unshaken anaerobic culture tube headspaces significantly inhibited (90% inhibition at 10% CO) both growth and hydrogen production by C. acetobutylicum. Growth was not sensitive to low partial pressures of CO (i.e., up to 15%) in pH-controlled fermentors (pH 4.5) that were continuously gassed and mixed. CO addition dramatically altered the glucose fermentation balance of C. acetobutylicum by diverting carbon and electrons away from H2, CO2, acetate, and butyrate production and towards production of ethanol and butanol. The butanol concentration was increased from 65 to 106 mM and the butanol productivity (i.e., the ratio of butanol produced/total acids and solvents produced) was increased by 31% when glucose fermentations maintained at pH 4.5 were continuously gassed with 85% N2-15% CO versus N2 alone. The results are discussed in terms of metabolic regulation of C. acetobutylicum saccharide fermentations to achieve maximal butanol or solvent yield.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J Bacteriol. 1980 Nov;144(2):569–578. doi: 10.1128/jb.144.2.569-578.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Blaschek H. P. Butanol Production by a Butanol-Tolerant Strain of Clostridium acetobutylicum in Extruded Corn Broth. Appl Environ Microbiol. 1983 Mar;45(3):966–973. doi: 10.1128/aem.45.3.966-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitdemange H., Cherrier C., Raval R., Gay R. Regulation of the NADH and NADPH-ferredoxin oxidoreductases in clostridia of the butyric group. Biochim Biophys Acta. 1976 Feb 24;421(2):334–337. doi: 10.1016/0304-4165(76)90300-7. [DOI] [PubMed] [Google Scholar]
- Schink B., Lupton F. S., Zeikus J. G. Radioassay for hydrogenase activity in viable cells and documentation of aerobic hydrogen-consuming bacteria living in extreme environments. Appl Environ Microbiol. 1983 May;45(5):1491–1500. doi: 10.1128/aem.45.5.1491-1500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]


