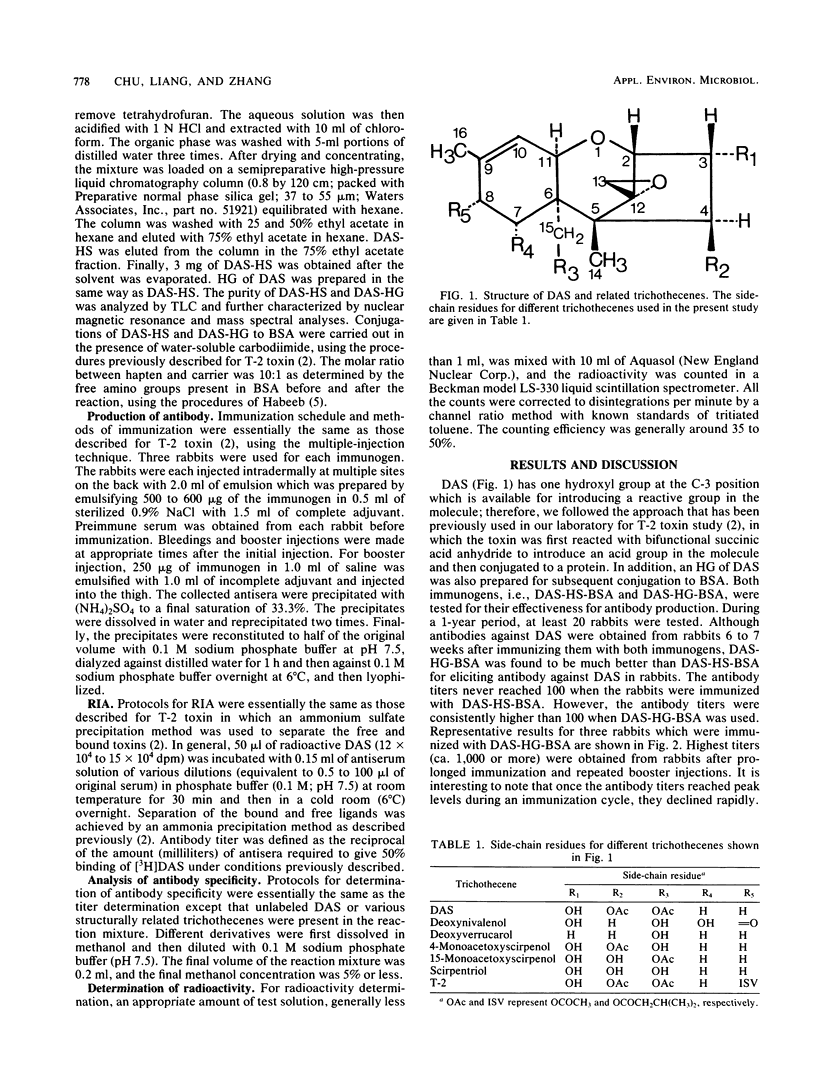
Abstract
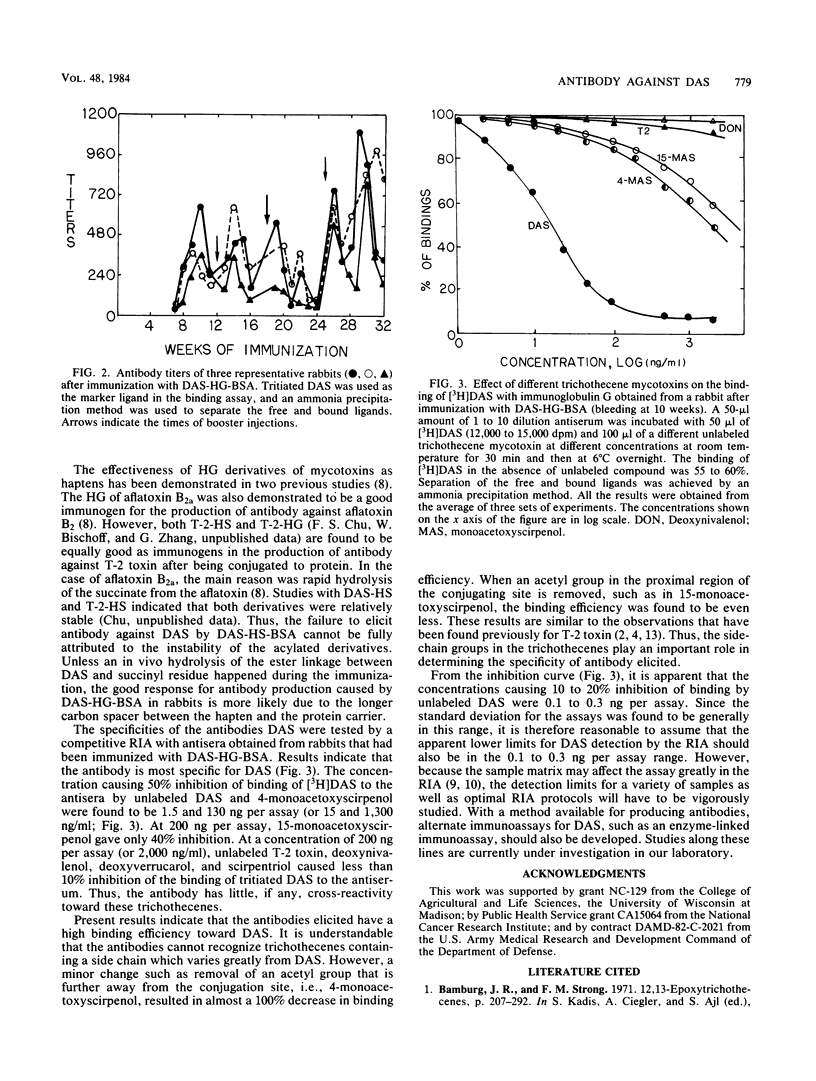
Antibodies against diacetoxyscirpenol (DAS) were obtained from rabbits after immunizing them with hemisuccinate or hemiglutarate derivatives of DAS conjugated to bovine serum albumin (BSA). DAS-hemiglutarate-BSA was found to be a much better immunogen than DAS-hemisuccinate-BSA. Competitive radioimmunoassay revealed that the antisera obtained from rabbits after immunization with DAS-hemiglutarate-BSA showed high specificity toward DAS. The concentrations causing 50% displacement of radioactive DAS by unlabeled DAS, 4-monoacetoxyscirpenol (MAS), and 15-MAS were found to be 1.5, 130, and 300 ng per assay, respectively. Thus, the cross-reactivities for 4-MAS and 15-MAS are ca. 87 and 300 times weaker than that of DAS. Practically no cross-reaction (less than 5% displacement) was observed when deoxynivalenol, T-2 toxin, deoxyverrucarol, and scirpentriol were tested at a concentration of 2,000 ng/ml.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu F. S., Grossman S., Wei R. D., Mirocha C. J. Production of antibody against T-2 toxin. Appl Environ Microbiol. 1979 Jan;37(1):104–108. doi: 10.1128/aem.37.1.104-108.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontelo P. A., Beheler J., Bunner D. L., Chu F. S. Detection of T-2 toxin by an improved radioimmunoassay. Appl Environ Microbiol. 1983 Feb;45(2):640–643. doi: 10.1128/aem.45.2.640-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Jarvis B. B., Stahly G. P., Pavanasasivam G., Mazzola E. P. Antileukemic compounds derived from the chemical modification of macrocyclic trichothecenes. 1. Derivatives of verrucarin A. J Med Chem. 1980 Sep;23(9):1054–1058. doi: 10.1021/jm00183a018. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Schmitz H., Essery J. M., Rose W., Howell H. G., O'Herron F. A., Nachfolger S., Huftalen J., Bradner W. T., Partyka R. A. Structural modifications of anguidin and antitumor activities of its analogues. J Med Chem. 1982 May;25(5):579–589. doi: 10.1021/jm00347a018. [DOI] [PubMed] [Google Scholar]
- Lee S., Chu F. S. Radioimmunoassay of T-2 toxin in biological fluids. J Assoc Off Anal Chem. 1981 May;64(3):684–688. [PubMed] [Google Scholar]
- Lee S., Chu F. S. Radioimmunoassay of T-2 toxin in corn and wheat. J Assoc Off Anal Chem. 1981 Jan;64(1):156–161. [PubMed] [Google Scholar]
- Peters H., Dierich M. P., Dose K. Enzyme-linked immunosorbent assay for detection of T-2 toxin. Hoppe Seylers Z Physiol Chem. 1982 Dec;363(12):1437–1441. doi: 10.1515/bchm2.1982.363.2.1437. [DOI] [PubMed] [Google Scholar]
- Wallace E. M., Pathre S. V., Mirocha C. J., Robison T. S., Fenton S. W. Synthesis of radiolabeled T-2 toxin. J Agric Food Chem. 1977 Jul-Aug;25(4):836–838. doi: 10.1021/jf60212a058. [DOI] [PubMed] [Google Scholar]
- Wei R., Strong F. M., Smalley E. B., Schnoes H. K. Chemical interconversion of T-2 and HT-2 toxins and related compounds. Biochem Biophys Res Commun. 1971 Oct 15;45(2):396–401. doi: 10.1016/0006-291x(71)90832-1. [DOI] [PubMed] [Google Scholar]


