Abstract
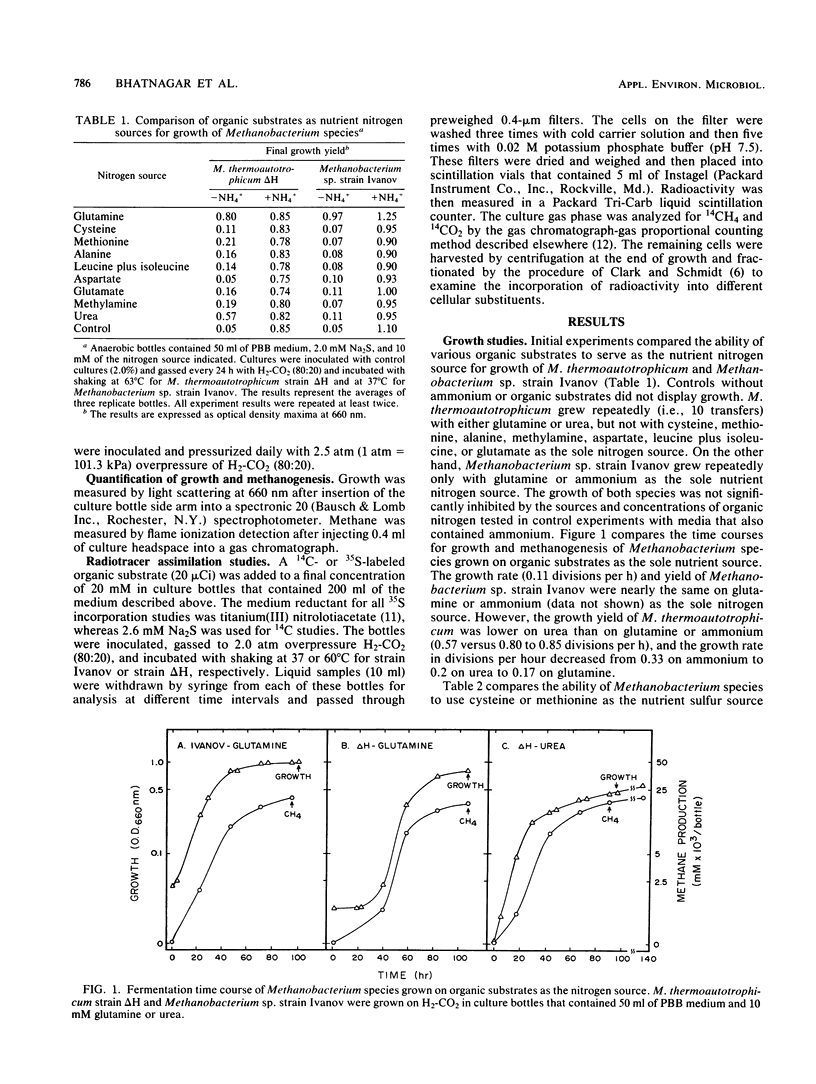
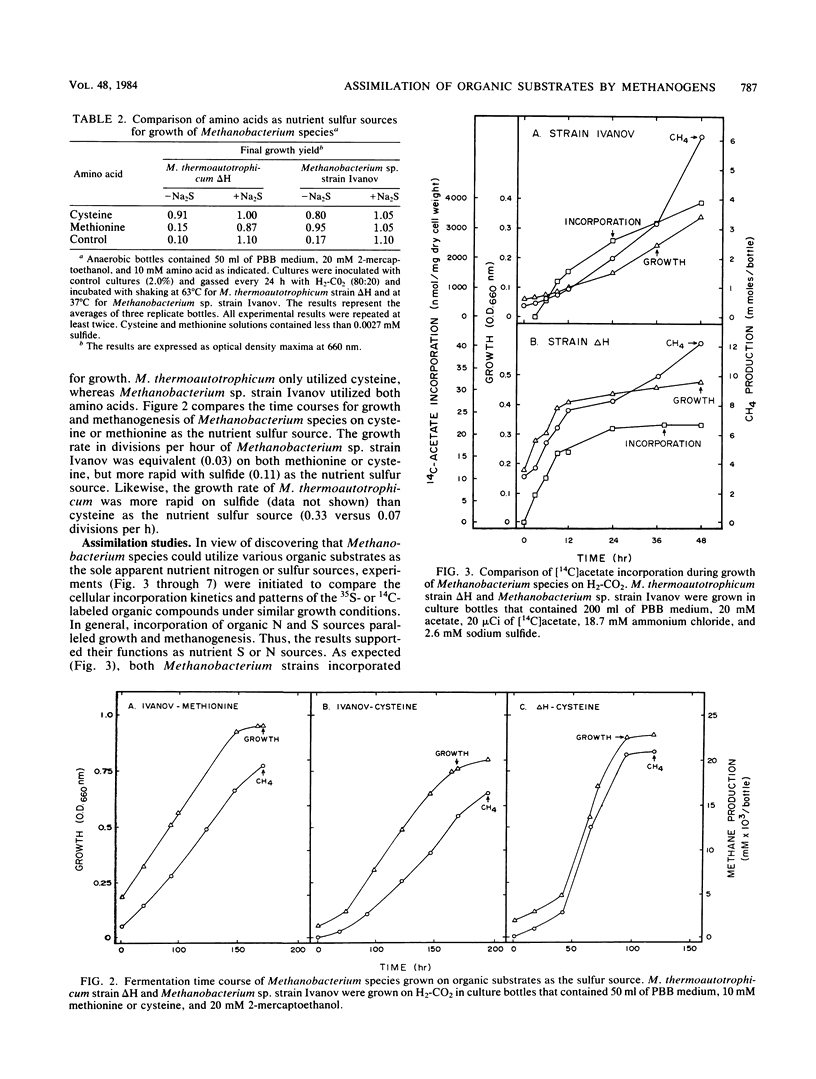
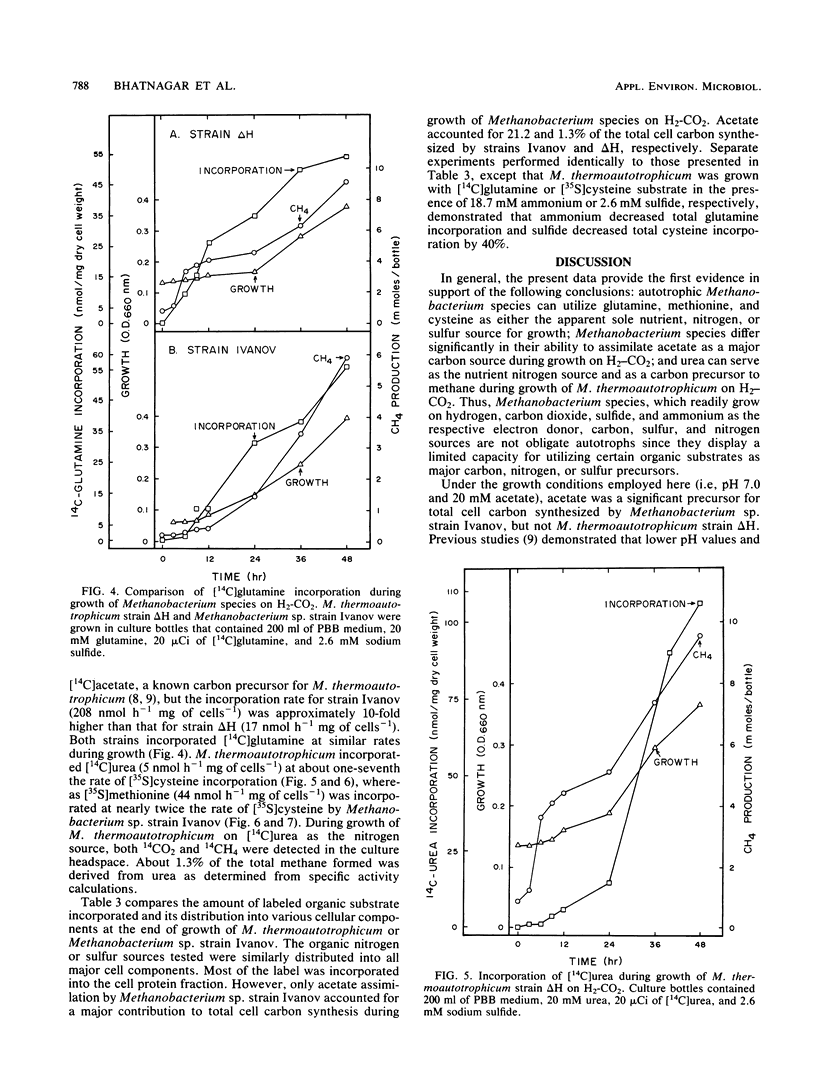
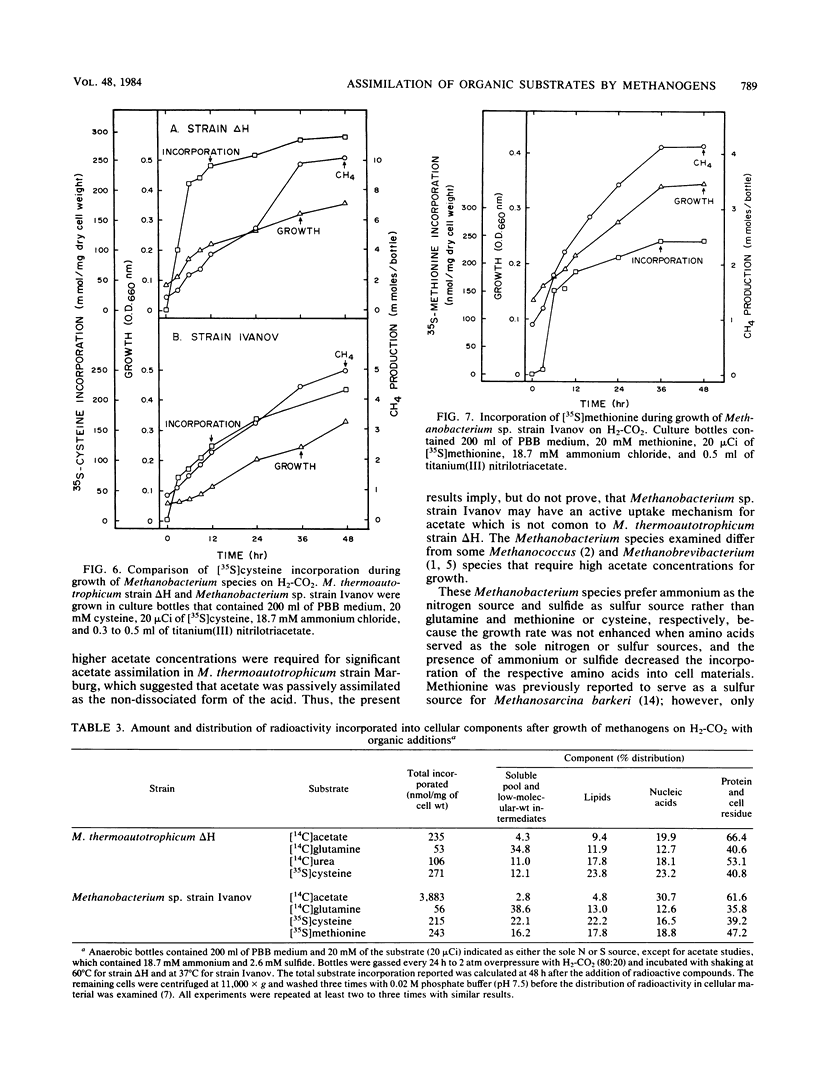
Experiments document the ability of two species of autotrophic methanogens to assimilate and utilize organic substrates as the nutrient sulfur or nitrogen source and as a carbon source during growth on H2-CO2. Methanobacterium thermoautotrophicum strain ΔH and the mesophilic species Methanobacterium sp. strain Ivanov grew with glutamine as the nitrogen source or cysteine as the sulfur source. M. thermoautotrophicum also utilized urea as the nitrogen source and as a carbon precursor for methane and cell synthesis. Methanobacterium sp. strain Ivanov grew with methionine as the sulfur source. The growth rate of two different Methanobacterium species was lower on an organic N or S source than on ammonium or sulfide. 35S and 14C tracer studies demonstrated that amino acid or urea assimilation correlated with time and amount of growth. The rate of [35S]cysteine incorporation was similar in strain ΔH (34 nmol h−1 mg of cells−1) and strain Ivanov (23 nmol h−1 mg of cells−1). However, the rate of [14C]acetate incorporation was dramatically different (17 versus 208 nmol h−1 mg of cells−1 in strains ΔH and Ivanov, respectively). [14C]acetate accounted for 1.3 and 21.2% of the total cell carbon synthesized by strains ΔH and Ivanov, respectively. Amino acids and urea were mainly assimilated into the cell protein fraction, but accounted for less than 2.0% of the total cell carbon synthesized. The data suggest that a biochemical-genetic approach to understanding cell carbon synthesis in methanogens is feasible; mutants that are auxotrophic for either acetate, glutamine, cysteine, or methionine are suggested as future targets for genetic studies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev S. S., Wolkin R., Kenealy W. R., Deniro M. J., Epstein S., Zeikus J. G. Methanogenic bacteria from the bondyuzhskoe oil field: general characterization and analysis of stable-carbon isotopic fractionation. Appl Environ Microbiol. 1983 Feb;45(2):691–697. doi: 10.1128/aem.45.2.691-697.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Growth response of Nitrosomonas europaea to amino acids. J Bacteriol. 1967 Apr;93(4):1302–1308. doi: 10.1128/jb.93.4.1302-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E., Thauer R. K. Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Apr 27;117(1):61–66. doi: 10.1007/BF00689352. [DOI] [PubMed] [Google Scholar]
- Kenealy W. R., Thompson T. E., Schubert K. R., Zeikus J. G. Ammonia assimilation and synthesis of alanine, aspartate, and glutamate in Methanosarcina barkeri and Methanobacterium thermoautotrophicum. J Bacteriol. 1982 Jun;150(3):1357–1365. doi: 10.1128/jb.150.3.1357-1365.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Trun N. J., Hamilton P. T. Beginning genetics with methanogens. Basic Life Sci. 1982;19:233–244. doi: 10.1007/978-1-4684-4142-0_19. [DOI] [PubMed] [Google Scholar]
- Taylor G. T., Pirt S. J. Nutrition and factors limiting the growth of a methanogenic bacterium (Methanobacterium thermoautotrophicum). Arch Microbiol. 1977 May 13;113(1-2):17–22. doi: 10.1007/BF00428574. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Metabolism of one-carbon compounds by chemotrophic anaerobes. Adv Microb Physiol. 1983;24:215–299. doi: 10.1016/s0065-2911(08)60387-2. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


