Abstract
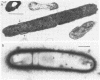
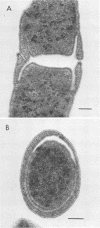
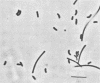
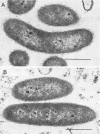
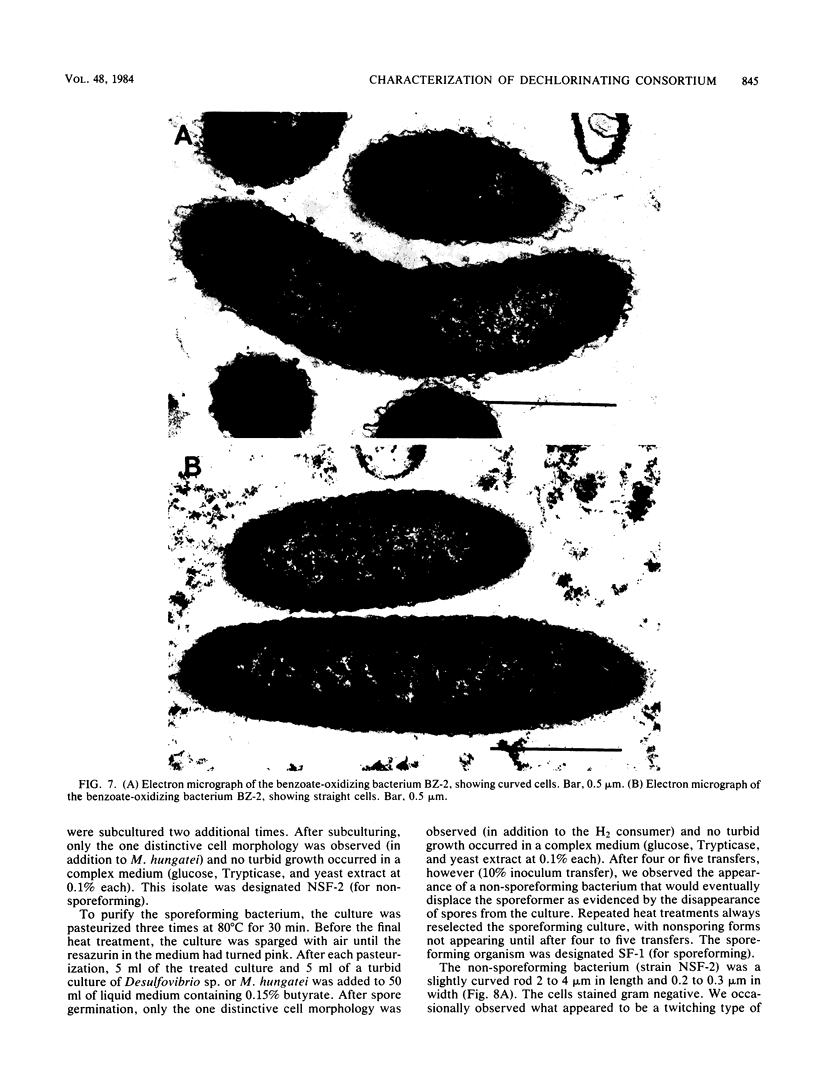
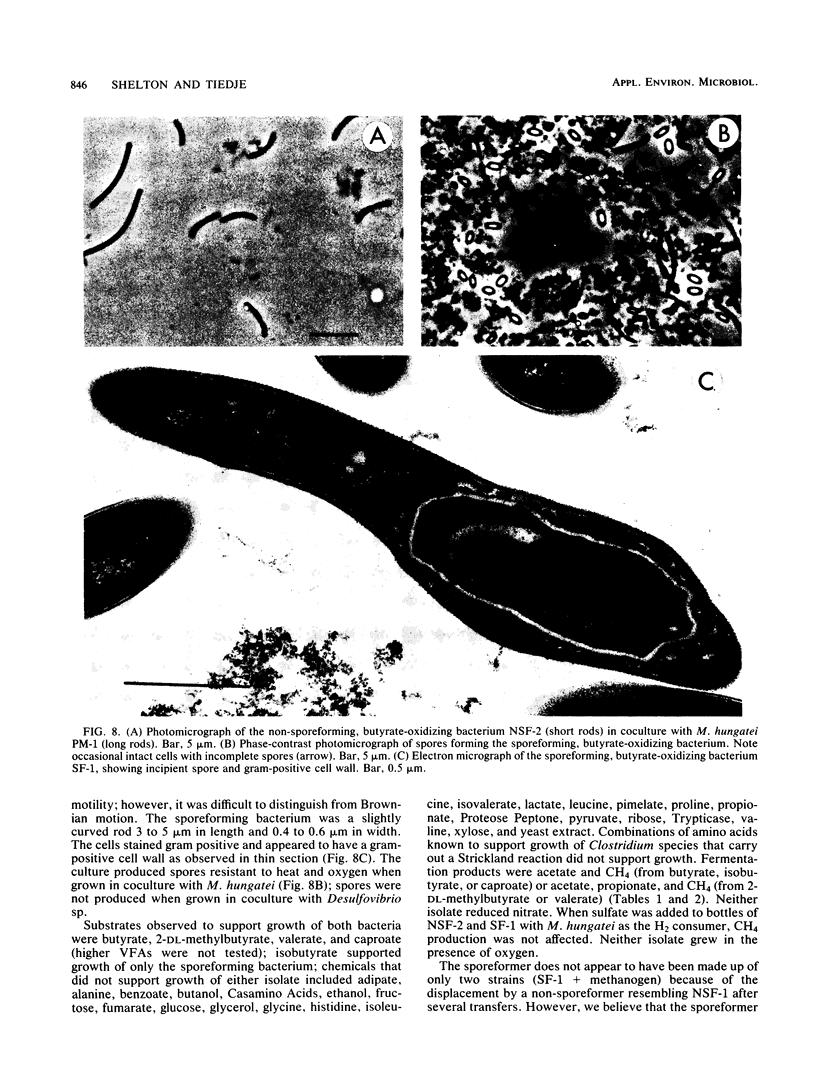
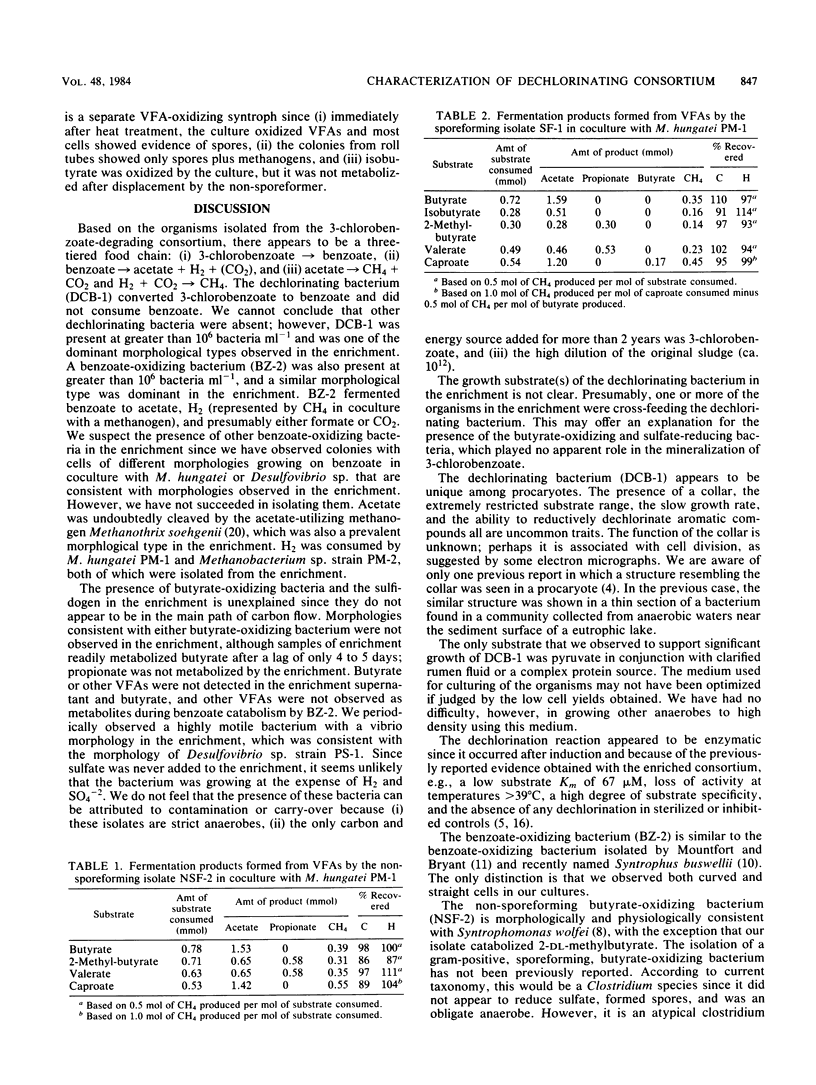
A methanogenic consortium able to use 3-chlorobenzoic acid as its sole energy and carbon source was enriched from anaerobic sewage sludge. Seven bacteria were isolated from the consortium in mono- or coculture. They included: one dechlorinating bacterium (strain DCB-1), one benzoate-oxidizing bacterium (strain BZ-2), two butyrate-oxidizing bacteria (strains SF-1 and NSF-2), two H2-consuming methanogens (Methanospirillum hungatei PM-1 and Methanobacterium sp. strain PM-2), and a sulfate-reducing bacterium (Desulfovibrio sp. strain PS-1). The dechlorinating bacterium (DCB-1) was a gram-negative, obligate anaerobe with a unique “collar” surrounding the cell. A medium containing rumen fluid supported minimal growth; pyruvate was the only substrate found to increase growth. The bacterium had a generation time of 4 to 5 days. 3-Chlorobenzoate was dechlorinated stoichiometrically to benzoate, which accumulated in the medium; the rate of dechlorination was ca. 0.1 pmol bacterium−1 day−1. The benzoate-oxidizing bacterium (BZ-2) was a gram-negative, obligate anaerobe and could only be grown as a syntroph. Benzoate was the only substrate observed to support growth, and, when grown in coculture with M. hungatei, it was fermented to acetate and CH4. One butyrate-oxidizing bacterium (NSF-2) was a gram-negative, non-sporeforming, obligate anaerobe; the other (SF-1) was a gram-positive, sporeforming, obligate anaerobe. Both could only be grown as syntrophs. The substrates observed to support growth of both bacteria were butyrate, 2-dl-methylbutyrate, valerate, and caproate; isobutyrate supported growth of only the sporeforming bacterium (SF-1). Fermentation products were acetate and CH4 (from butyrate, isobutyrate, or caproate) or acetate, propionate, and CH4 (from 2-dl-methylbutyrate or valerate) when grown in coculture with M. hungatei. A mutualism among at least the dechlorinating, benzoate-oxidizing, and methane-forming members was apparently required for utilization of the 3-chlorobenzoate substrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd S. A., Shelton D. R. Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge. Appl Environ Microbiol. 1984 Feb;47(2):272–277. doi: 10.1128/aem.47.2.272-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R., Berry D., Tiedje J. M. Anaerobic biodegradation of phenolic compounds in digested sludge. Appl Environ Microbiol. 1983 Jul;46(1):50–54. doi: 10.1128/aem.46.1.50-54.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. E., Tiedje J. M. A morphological study of anaerobic bacteria from the hypolimnia of two Michigan lakes. Can J Microbiol. 1975 Mar;21(3):362–376. doi: 10.1139/m75-051. [DOI] [PubMed] [Google Scholar]
- Horowitz A., Suflita J. M., Tiedje J. M. Reductive dehalogenations of halobenzoates by anaerobic lake sediment microorganisms. Appl Environ Microbiol. 1983 May;45(5):1459–1465. doi: 10.1128/aem.45.5.1459-1465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick D., Biggs S. R., Conway B., Finn C. M., Hawkins D. R., Honda T., Ishida M., Powell G. P. Metabolism of N-(2,3-dichlorophenyl)-3,4,5,6-tetrachlorophthalamic acid (techlofthalam) in paddy soil and rice. J Agric Food Chem. 1981 Nov-Dec;29(6):1149–1153. doi: 10.1021/jf00108a012. [DOI] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy N. B., Kaufman D. D., Fries G. F. Degradation of pentachlorophenol (PCP) in aerobic and anaerobic soil. J Environ Sci Health B. 1979;14(1):1–14. doi: 10.1080/03601237909372110. [DOI] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. General method for determining anaerobic biodegradation potential. Appl Environ Microbiol. 1984 Apr;47(4):850–857. doi: 10.1128/aem.47.4.850-857.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suflita J. M., Horowitz A., Shelton D. R., Tiedje J. M. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science. 1982 Dec 10;218(4577):1115–1117. doi: 10.1126/science.218.4577.1115. [DOI] [PubMed] [Google Scholar]
- Suflita J. M., Robinson J. A., Tiedje J. M. Kinetics of microbial dehalogenation of haloaromatic substrates in methanogenic environments. Appl Environ Microbiol. 1983 May;45(5):1466–1473. doi: 10.1128/aem.45.5.1466-1473.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Zehnder A. J., Huser B. A., Brock T. D., Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980 Jan;124(1):1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]