Abstract
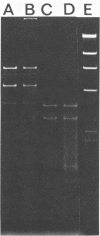
Toxigenic Clostridium botulinum and nontoxigenic C. sporogenes, C. subterminale, and C. botulinum-like organisms from a variety of sources were screened for plasmids. Of the 68 toxigenic C. botulinum isolates, 56% carried one or more plasmids, ranging in mass from 2.1 to 81 megadaltons. Within individual groups (based on the type of neurotoxin produced), many strains showed identical plasmid banding patterns on agarose gels. Of the 15 nontoxigenic strains tested, 40% also carried one or more plasmids ranging from 1.7 to 25.0 megadaltons, with both unique and common banding patterns represented. A total of 67 plasmids from both toxigenic and nontoxigenic strains were detected. At this time, no phenotypic functions have been assessed for these plasmids, and they must therefore be considered cryptic. A variety of lysing and extraction techniques were necessary to detect plasmids in the different C. botulinum groups.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschek H. P., Solberg M. Isolation of a plasmid responsible for caseinase activity in Clostridium perfringens ATCC 3626B. J Bacteriol. 1981 Jul;147(1):262–266. doi: 10.1128/jb.147.1.262-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefort G., Magot M., Ionesco H., Sebald M. Characterization and transferability of Clostridium perfringens plasmids. Plasmid. 1977 Nov;1(1):52–66. doi: 10.1016/0147-619x(77)90008-7. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T. Interconversion of type C and D strains of Clostridium botulinum by specific bacteriophages. Appl Microbiol. 1974 Jan;27(1):251–258. doi: 10.1128/am.27.1.251-258.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Wieler D. I. Characteristics of Clostridium botulinum type F isolated from the Pacific Coast of the United States. Appl Microbiol. 1967 Nov;15(6):1316–1323. doi: 10.1128/am.15.6.1316-1323.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez D. F., Ciccarelli A. S. New strains of Clostridium botulinum subtype Af. Zentralbl Bakteriol Orig A. 1978 Apr;240(2):215–220. [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Iida H. Phage-conversion of toxigenicity in Clostridium botulinum types C and D. Jpn J Med Sci Biol. 1971 Feb;24(1):53–56. [PubMed] [Google Scholar]
- Kautter D. A., Harmon S. M., Lynt R. K., Jr, Lilly T., Jr Antagonistic effect on Clostridium botulinum type E by organisms resembling it. Appl Microbiol. 1966 Jul;14(4):616–622. doi: 10.1128/am.14.4.616-622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritani K., Mitsui N., Nakamura S., Nishida S. Numerical taxonomy of Clostridium botulinum and Clostridium sporogenes strains, and their susceptibilities to induced lysins and to mitomycin C. Jpn J Microbiol. 1973 Sep;17(5):361–372. doi: 10.1111/j.1348-0421.1973.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Schnepf H. E., Whiteley H. R. Diversity of locations for Bacillus thuringiensis crystal protein genes. J Bacteriol. 1983 Apr;154(1):419–428. doi: 10.1128/jb.154.1.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W. J., Aaronson W., Silver R. P., Habig W. H., Hardegree M. C. Plasmid-associated toxigenicity in Clostridium tetani. J Infect Dis. 1980 Oct;142(4):623–623. doi: 10.1093/infdis/142.4.623. [DOI] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Rapid screening procedure for detection of plasmids in streptococci. J Bacteriol. 1979 Dec;140(3):1112–1115. doi: 10.1128/jb.140.3.1112-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Riemann H. Correlation of toxic and non-toxic strains of Clostridium botulinum by DNA composition and homology. J Gen Microbiol. 1970 Jan;60(1):117–123. doi: 10.1099/00221287-60-1-117. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Riemann H. The genetic relatedness of proteolytic Clostridium botulinum strains. J Gen Microbiol. 1970 Nov;64(1):85–90. doi: 10.1099/00221287-64-1-85. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Okado I., Nakashio S., Nishida S. Clostridium sporogenes isolates and their relationship to C. botulinum based on deoxyribonucleic acid reassociation. J Gen Microbiol. 1977 Jun;100(2):395–401. doi: 10.1099/00221287-100-2-395. [DOI] [PubMed] [Google Scholar]
- Oguma K., Iida H., Inoue K. Bacteriophage and toxigenicity in Clostridium botulinum: an additional evidence for phage conversion. Jpn J Microbiol. 1973 Sep;17(5):425–426. doi: 10.1111/j.1348-0421.1973.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Pan-Hou H. S., Hosono M., Imura N. Plasmid-controlled mercury biotransformation by Clostridium cochlearium T-2. Appl Environ Microbiol. 1980 Dec;40(6):1007–1011. doi: 10.1128/aem.40.6.1007-1011.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Maher E. A., Somers E. B., Campos E., Duncan C. L. Isolation and characterization of multiply antibiotic-resistant Clostridum perfringens strains from porcine feces. Antimicrob Agents Chemother. 1978 May;13(5):871–880. doi: 10.1128/aac.13.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Scott V. N., Duncan C. L. Identification of a transferable tetracycline resistance plasmid (pCW3) from Clostridium perfringens. Plasmid. 1978 Sep;1(4):563–570. doi: 10.1016/0147-619x(78)90013-6. [DOI] [PubMed] [Google Scholar]
- Schiller J., Groman N., Coyle M. Plasmids in Corynebacterium diphtheriae and diphtheroids mediating erythromycin resistance. Antimicrob Agents Chemother. 1980 Nov;18(5):814–821. doi: 10.1128/aac.18.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J., Strom M., Groman N., Coyle M. Relationship between pNG2, an Emr plasmid in Corynebacterium diphtheriae, and plasmids in aerobic skin coryneforms. Antimicrob Agents Chemother. 1983 Dec;24(6):892–901. doi: 10.1128/aac.24.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strider W., Camien M. N., Warner R. C. Renaturation of denatured, covalently closed circular DNA. J Biol Chem. 1981 Aug 10;256(15):7820–7829. [PubMed] [Google Scholar]
- Sugiyama H. Clostridium botulinum neurotoxin. Microbiol Rev. 1980 Sep;44(3):419–448. doi: 10.1128/mr.44.3.419-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. I., Riemann H., Lee W. H. Thermal stability of the deoxyribonucleic acid hybrids between the proteolytic strains of Clostridium botulinum and Clostridium sporogenes. Can J Microbiol. 1972 Jan;18(1):97–99. doi: 10.1139/m72-016. [DOI] [PubMed] [Google Scholar]