Abstract
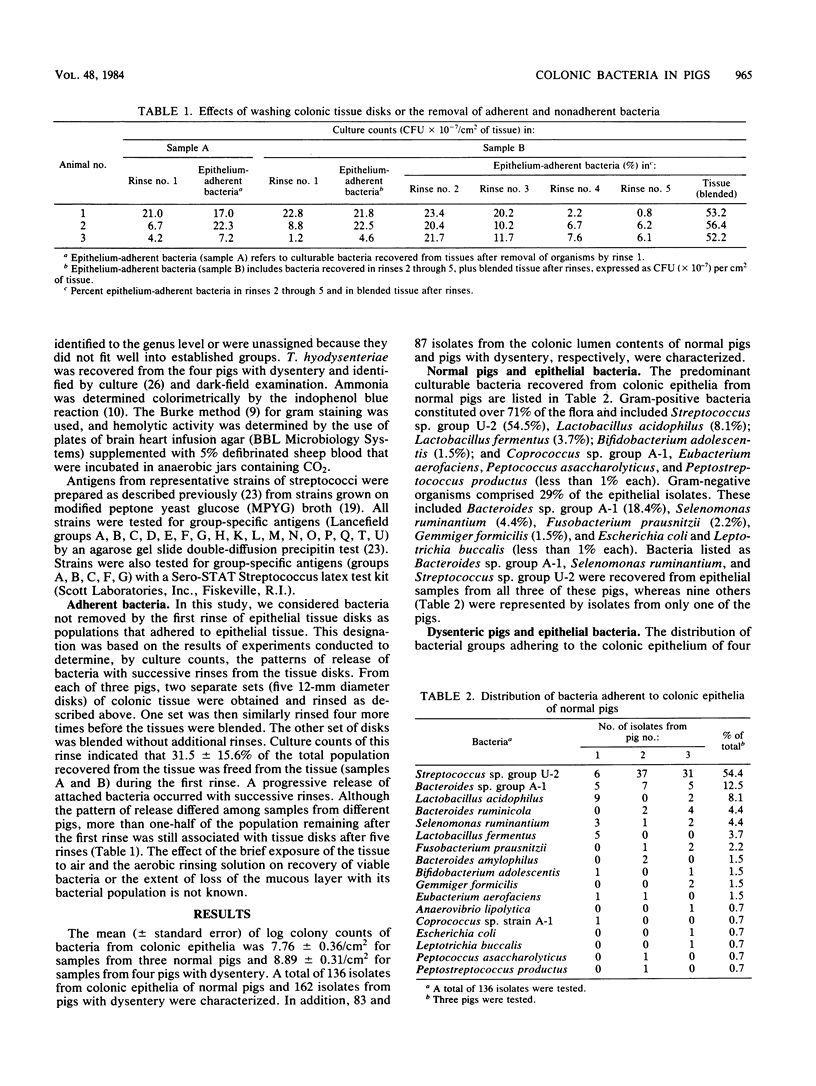
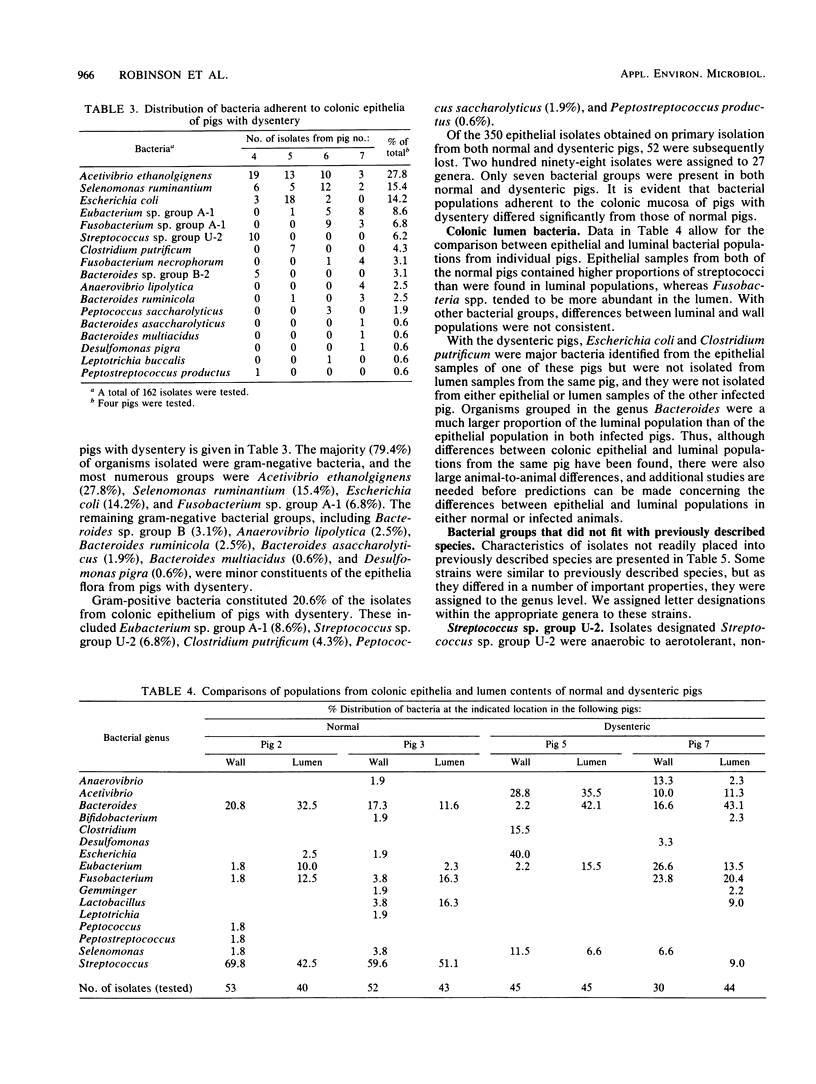
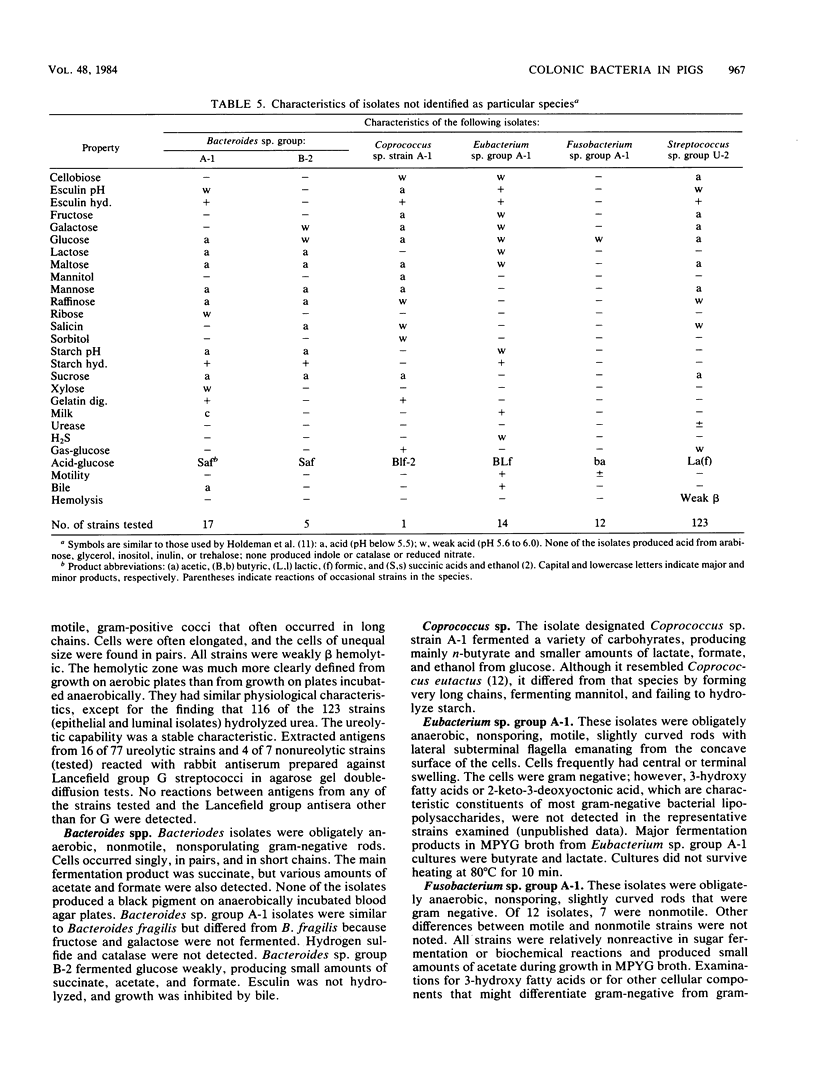
Bacterial populations adherent to the mucosa of the proximal colons of weaned, healthy pigs were compared with populations from pigs with dysentery induced by inoculation with a culture of Treponema hyodysenteriae. Isolates (136) representative of the predominant flora adherent to colonic epithelia of normal pigs and isolates (162) from pigs with dysentery were cultured anaerobically on a rumen fluid-based medium and characterized. Most (71%) of the isolates from colonic epithelia of normal pigs were gram positive, whereas 88% of the epithelia-associated isolates from pigs with dysentery were gram negative. The geometric mean of colony counts was 5.7 X 10(7)/cm2 of colonic tissue from three normal pigs and 7.7 X 10(8)/cm2 from four pigs with dysentery. A number of isolates obtained from contents of the lumens of normal pigs with dysentery were also characterized. Comparison of isolates from epithelial tissue and from contents of the lumens of the same pig indicated that these populations were different. Our results indicate that physiological changes that occur in the colons of pigs with dysentery are accompanied by marked changes in the microbial populations in the colons. The factors which regulate the population changes are not yet understood.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalbaek B. Gram-negative anaerobes in the intestinal flora of pigs. Acta Vet Scand. 1972;13(2):228–237. doi: 10.1186/BF03548576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., Robinson I. M., Bucklin J. A., Booth G. D. Comparison of bacterial populations of the pig cecum and colon based upon enumeration with specific energy sources. Appl Environ Microbiol. 1979 Jun;37(6):1142–1151. doi: 10.1128/aem.37.6.1142-1151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere J., Henderickx H. K., Vervaeke I. Influence of nutritional doses of virginiamycin and spiramycin on the quantitative and topographical composition of the gastro-intestinal flora of artificially reared piglets. Zentralbl Bakteriol Orig A. 1973 Mar;223(2):348–355. [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L. Growth in Treponema hyodysenteriae in liquid medium. Vet Rec. 1974 Sep 7;95(10):219–220. doi: 10.1136/vr.95.10.219. [DOI] [PubMed] [Google Scholar]
- McAllister J. S., Kurtz H. J., Short E. C., Jr Changes in the intestinal flora of young pigs with postweaning diarrhea or edema disease. J Anim Sci. 1979 Sep;49(3):868–879. doi: 10.2527/jas1979.493868x. [DOI] [PubMed] [Google Scholar]
- Rall G. D., Wood A. J., Wescott R. B., Dommert A. R. Distribution of bacteria in feces of swine. Appl Microbiol. 1970 Nov;20(5):789–792. doi: 10.1128/am.20.5.789-792.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J., Bucklin J. A. Characterization of the cecal bacteria of normal pigs. Appl Environ Microbiol. 1981 Apr;41(4):950–955. doi: 10.1128/aem.41.4.950-955.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. G. Types and distribution of anaerobic bacteria in the large intestine of pigs. Appl Environ Microbiol. 1979 Feb;37(2):187–193. doi: 10.1128/aem.37.2.187-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. W., JONES J. E. OBSERVATIONS ON THE ALIMENTARY TRACT AND ITS BACTERIAL FLORA IN HEALTHY AND DISEASED PIGS. J Pathol Bacteriol. 1963 Oct;86:387–412. [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirhead P. A. Isolation and identification of fecal bacteria from adult swine. Appl Environ Microbiol. 1977 Jan;33(1):79–84. doi: 10.1128/aem.33.1.79-84.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman R. D., Nord N. A. Serologic group relationship of P and U streptococci. Cornell Vet. 1974 Jul;64(3):376–386. [PubMed] [Google Scholar]
- Sinkovics G. Therapeutic experiments and intestinal flora studies in swine dysentery. Zentralbl Veterinarmed B. 1974 Sep;21(9):706–714. doi: 10.1111/j.1439-0450.1974.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Songer J. G., Kinyon J. M., Harris D. L. Selective medium for isolation of Treponema hyodysenteriae. J Clin Microbiol. 1976 Jul;4(1):57–60. doi: 10.1128/jcm.4.1.57-60.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp S. C., Robinson I. M., Harris D. L., Glock R. D., Matthews P. J., Alexander T. J. Pathogenic synergism between Treponema hyodysenteriae and other selected anaerobes in gnotobiotic pigs. Infect Immun. 1979 Dec;26(3):1042–1047. doi: 10.1128/iai.26.3.1042-1047.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


