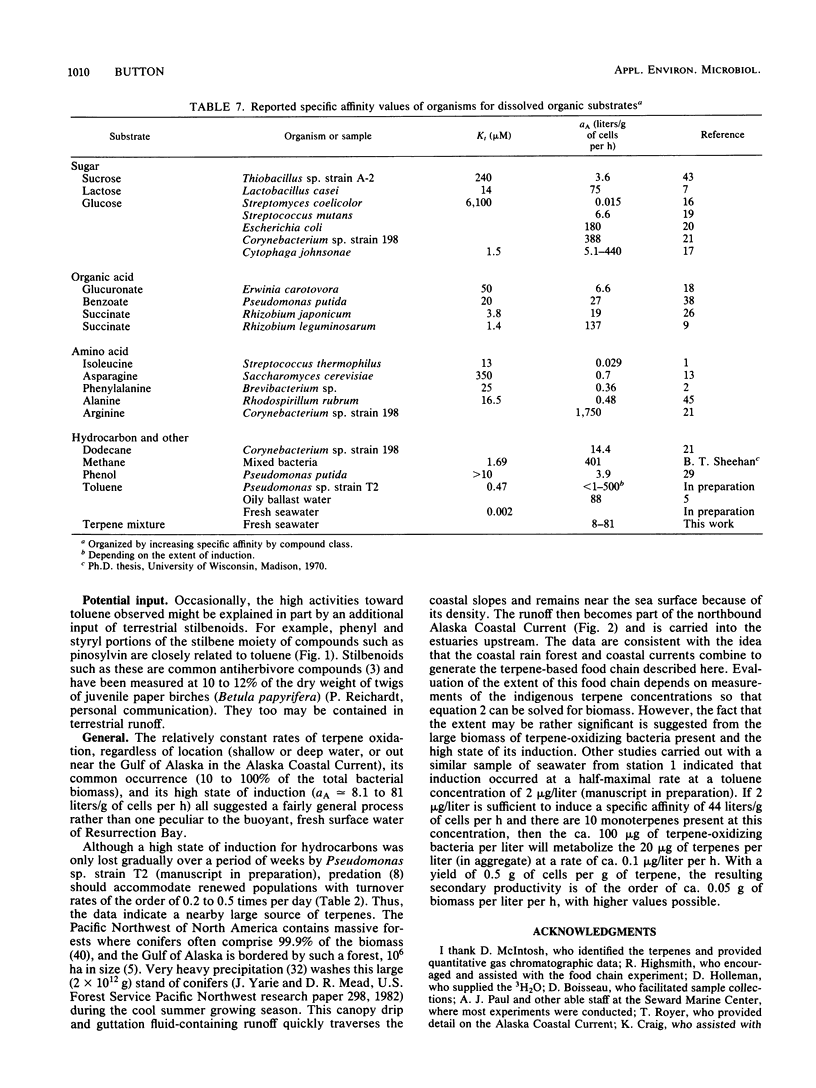
Abstract
A mixture of 14C-terpenes was prepared from conifer seedlings and introduced into fresh seawater samples taken near Seward, Alaska. Initial rates of oxidation by the indigenous bacteria were linear and faster than the rates of toluene oxidation. Turnover times were 4 to 19 days. Autoradiographic measurements with 3H-terpenes indicated that at least 10% of the 0.6 × 109 to 2.7 × 109 bacteria per liter present could catabolize terpenes. The rate of terpene oxidation, 24 μg of terpenes per g of cells per h with 3 μg of terpenes added per liter, was a constant function of bacterial biomass. The specific affinity of the process was estimated to be between 8.1 and 81 liters/g of cells per h, indicating a high state of induction and the probable presence of terpenes. Terpene-oxidizing bacteria were grown on [14C]alanine and added to fresh seawater samples. Transfer of the bacterial radioactivity into larger particles at a rate of 146 pg/liter per h from the 2.3 × 109 organisms added indicated that any terpenes present would participate in the food chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akpemado K. M., Bracquart P. A. Uptake of Branched-Chain Amino Acids by Streptococcus thermophilus. Appl Environ Microbiol. 1983 Jan;45(1):136–140. doi: 10.1128/aem.45.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyaval P., Moreira E., Desmazeaud M. J. Transport of aromatic amino acids by Brevibacterium linens. J Bacteriol. 1983 Sep;155(3):1123–1129. doi: 10.1128/jb.155.3.1123-1129.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J. P., Wieland G. D., Reichardt P. B., Lewis V. E., McCarthy M. C. Pinosylvin methyl ether deters snowshoe hare feeding on green alder. Science. 1983 Dec 2;222(4627):1023–1025. doi: 10.1126/science.222.4627.1023. [DOI] [PubMed] [Google Scholar]
- Button D. K., Robertson B. R., Craig K. S. Dissolved hydrocarbons and related microflora in a fjordal seaport: sources, sinks, concentrations, and kinetics. Appl Environ Microbiol. 1981 Oct;42(4):708–719. doi: 10.1128/aem.42.4.708-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Schell D. M., Robertson B. R. Sensitive and accurate methodology for measuring the kinetics of concentration-dependent hydrocarbon metabolism rates in seawater by microbial communities. Appl Environ Microbiol. 1981 Apr;41(4):936–941. doi: 10.1128/aem.41.4.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J Bacteriol. 1983 Jun;154(3):1403–1413. doi: 10.1128/jb.154.3.1403-1413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. J., Wadsö I. An equation of state describing hydrophobic interactions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2955–2958. doi: 10.1073/pnas.73.9.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Quesneau Y., Robert-Baudouy J. Aldohexuronate transport system in Erwinia carotovora. J Bacteriol. 1983 May;154(2):663–668. doi: 10.1128/jb.154.2.663-668.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfle M. G. Long-Term Changes in Chemostat Cultures of Cytophaga johnsonae. Appl Environ Microbiol. 1983 Nov;46(5):1045–1053. doi: 10.1128/aem.46.5.1045-1053.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C. W., Robrish S. A., Curtis M. A., Sharer S. A., Bowen W. H. Application of a competition model to the growth of Streptococcus mutans and Streptococcus sanguis in binary continuous culture. Appl Environ Microbiol. 1983 Apr;45(4):1277–1282. doi: 10.1128/aem.45.4.1277-1282.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Wang C. H. How close to the theoretical diffusion limit do bacterial uptake systems function? Arch Microbiol. 1982 Feb;131(1):36–42. doi: 10.1007/BF00451496. [DOI] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister C. F., Lepo J. E. Succinate transport by free-living forms of Rhizobium japonicum. J Bacteriol. 1983 Mar;153(3):1155–1162. doi: 10.1128/jb.153.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C. Structure-activity relationships in microbial transformation of phenols. Appl Environ Microbiol. 1982 Jul;44(1):153–158. doi: 10.1128/aem.44.1.153-158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol. 1982 Oct;44(4):945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. R., Wheelis M. L. Active transport of benzoate in Pseudomonas putida. J Gen Microbiol. 1982 Aug;128(8):1749–1753. doi: 10.1099/00221287-128-8-1749. [DOI] [PubMed] [Google Scholar]
- Waring R. H., Franklin J. F. Evergreen coniferous forests of the pacific northwest. Science. 1979 Jun 29;204(4400):1380–1386. doi: 10.1126/science.204.4400.1380. [DOI] [PubMed] [Google Scholar]
- Zebrower M., Loach P. A. Efficiency of light-driven metabolite transport in the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1982 Jun;150(3):1322–1328. doi: 10.1128/jb.150.3.1322-1328.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


